|
[Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]

Phosphorus and Iron Nutrition in Australian Native Plants
Simon Leake
The phenomenon of phosphorus toxicity in certain native species has been studied extensively in recent years. Plants in the Proteaceae, Rutaceae, some Fabaceae and Mimosaceae, some Myrtaceae and Haemodoraceae have been shown to exhibit problems. Most of these plants have become adapted to growing in very low P soils and have developed mechanisms to extract otherwise unavailable P.
It has been apparent for some years to growers of native plants and proteas that a specific toxicity is seen when these plants are supplied with excessive soil phosphorus. Symptoms of this toxicity are various but include lack of growth, apparent iron deficiency (interveinal chlorosis of youngest leaves), red colours starting in oldest leaves, drop of oldest leaves, tip necrosis in the worst cases, and a susceptibility to root rot fungi such as phytophthora. The condition can occur in pot culture and in soils and is often a compounding factor in transplant and environmental shock. Goodwin (1981) showed that plants not exhibiting symptoms could be made to produce symptoms under temperature or moisture stress.
The problem is not as simple as an excess of P and research has firmly established that this effect is influenced by other nutrient availability. Nichols (1988) showed that increased calcium levels can exacerbate toxicity and that extra N can reduce it. Goodwin (1981) showed that very high levels of N can exacerbate it, while Handreck (1991a and 1991b), Goodwin (1981), showed that sensitivity to high P levels can be reduced by improving iron supply. Our own experience is that P toxicity can occur in pots even with so called low P Native Plant Foods and in soils especially on old orchard soil improved over many years with superphosphate and fowl manure additions. We have also found that considerable control can be exerted over this toxicity by influencing iron supply.
The factors influencing iron supply in soils and growing media include-
- total soil iron levels
- soil pH
- soil organic matter
- soil P levels
- soil to root contact
The interactions of these phenomena result, for example, in iron deficiency in a beach sand heavily improved with organic matter. Beach sands are often calcareous (high pH) and are mostly low in iron (being highly siliceous and not possessing the red earth colours associated with high iron levels in most mineral soils). Increasing the organic matter levels in these soils can further reduce iron supply. Iron is insoluble at high pH due to the formation of hydroxides and carbonates. Iron deficiency in potting mixes is also common given the low iron content of organic matter (commonly pine bark and sawdust) and the fixation ability of solid organic matter.
Addition of high levels of phosphate to a soil results in an insoluble phosphate being formed-
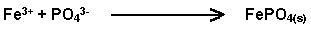 This is the same reaction resulting from rust converter being painted on rusty metal, phosphoric acid being the active ingredient. It is known from crop plants that high P levels can reduce Cu, Zn and Fe deficiencies. This is called a nutrient interaction.
This is the same reaction resulting from rust converter being painted on rusty metal, phosphoric acid being the active ingredient. It is known from crop plants that high P levels can reduce Cu, Zn and Fe deficiencies. This is called a nutrient interaction.
Research (Jenny 1980) has also showed the importance of direct and firm contact of the root with the soil. In one experiment, plants were grown in the presence and absence of iron oxide pellets in the soil and an alkaline nutrient solution (minus iron) recirculated between the pots. Pots in the absence of iron pellets showed deficiency whereas with pellets they did not. Where roots get close to iron surfaces they can collect iron which is not soluble. Plants poorly rooted in loose soil can thus show iron deficiency.
Inside the plant the same reaction of phosphate with iron can occur resulting in a depletion of iron reserves in the plant as iron phosphate is laid down mostly in the stem tissue and at the margins of leaves. With meagre iron supply already, the uptake of excessive phosphate levels can result in an acute deficiency of iron. Where the plants in question are "iron inefficient", that is they are adapted to abundant iron supply (see Handreck 1991a), the problem is often worse and Australian and South African proteaceae are the most susceptible groups of plant being both P sensitive and iron inefficient.
Theory should suggest then, that supplying the plant with abundant iron may help to overcome some of the physiological problems associated with excessive P levels in susceptible plants. Research by Goodwin (1981), Nichols (1988) and Handreck (1991a and 1991b) has shown this to be the case. In a case of severe phosphate toxicity documented by ourselves, Proteas were grown on old orchard land showing very elevated soil P availability. Plants exhibited classic P toxicity symptoms of redness in margins of young and old foliage, poor vigour and flowering, and interveinal chlorosis on young foliage. The use of iron both as a foliar chelate application and soil application of ferrous sulphate controlled symptoms with regular use and has gradually resulted in lowered soil P availability. With time it appears that less and less frequent application of the iron may be needed to obtain control, and this offers the hope that eventually the condition will be fully cured.
Handreck (1991b) produced a table illustrating Fe requirements given a range of P levels, and this also illustrates the plant species showing these problems.
Goodwin (1981) produced a list of plants known to be sensitive to phosphorus. This is shown in Table 2. Note that there are some minor conflicts between these lists and this illustrates the conditional nature of the expression of P toxicity symptoms. We have found definite P sensitivity and iron inefficiency in Macadamia integrifolia and definite improvement in foliar growth and nut yields with use of iron sprays and soil iron and sulphur applications.
Table 1- Maximum concentrations of P (ppm) tolerated by species growing in a soil-less potting medium at two levels of extractable Fe (Handreck 1991a)
| Fe (ppm) in the mix | Species |
| 34 | 19 |
| 3 | <3 | Acacia merrallii, Grevillea leucopteris, Hakea bucculenta, H.francisiana, H.petiolaris |
| 5 | <3 | Acacia imbricata, Banksia benthamiana, B.brownii, B.lemanniana, B.leptophylla, B.sphaerocarpa, Grevillea banksii, Hakea salicifolia |
| 5 | 3 | Acacia baileyana, A.decurrens, A.spectabilis, Hakea sericea |
| 8 | 7 | Acacia dealbata, A.glaucoptera, A.ligulata, A.lineata, A.montana, A.myrtifolia, A.retinoides, Hakea laurina |
| 11 | 3 | Banksia tricuspis, Hakea rostrata |
| 11 | 10 | Acacia argyrophylla, A.baileyana purpurea, A.burkittii, A.calamifolia, A.florabunda, A.iteaphylla, A.menzelii, A.microcarpa, A.papyrocarpa, A.paradoxa, A.rigens, A.rivalis, A.rotundifolia, A.sclerophylla, Banksia aculeata, B.laricina, B.speciosa, Grevillea intricata, G.robusta, Hakea suberea |
| >20 | 14 | Acacia cyclops, A.fimbriata, A.hakeoides, A.longifolia sophorae, A.melanoxylon, A.nyssophylla, A.pendula, A.ramulosa, Hakea muelleriana |
| >20 | >25 | Acacia longifolia, A.saligna, A.truncata, A.victoriae, Hakea leucoptera |
Table 2 - Known Phosphorus Sensitive Plants (Goodwin 1981)
Acacia baileyana, A.iteaphylla, A.obtusata, A.sauveolens, A.verticillata
Banksia aemula, B.ericifolia, B.oblongifolia, B.robur
Beaufortia squarrosa
Boronia megastigma
Callistemon citrinus
Grevillea aquifolium, G.glabella, G."Poorinda Firebird"
Hakea laurina
Protea longifolia *, P.macrocephala*
Pultenaea pedunculata
Telopea speciosissima |
* Not Australian species
Fertilising Australian Plants
As a general rule, fertilisers can be used to speed the growth of potted and soil grown P sensitive plants. It is also true that the great majority of native plants are not sensitive to high P levels and will respond well to added phosphorus. However, since the lists of known sensitive plants are probably incomplete, caution should be exercised in using fertilisers and the following could be taken as a guide to such use.
1. Soil
Fertilisers low in water soluble P and low in total P are to be preferred. Fertilisers supplying P in the form of bone are routinely use by Protea growers with few ill effects, however some caution should be used with regular long term use as the total P level in Blood and Bone is quite high (5%) and harmful levels may accumulate. Various native plant fertilisers are available and the analysis of these should be checked. P should be supplied as insoluble forms only in these formulations. A suitable home made mixture might be:
| Sulphate of ammonium | 1 part |
| Blood and Bone | 1 part |
| Sulphate of potash | 1 part |
| Sulphate of iron | 1 part |
As a general rule avoid fowl manure and mushroom compost altogether and make sure that any fertiliser used has an NPK ratio something like 8:1:5.
Use chelated foliar sprays to mask or correct symptoms in the short term. To try to correct the soil problem in the long term use soil applications of sulphate of iron where iron content in the soil is low, or pH is high (alkaline) due to excessive lime levels. On naturally limey (alkaline) and very sandy or organic soils, continued use of foliar iron chelates may be needed to grow certain very susceptible plants.
2. Potting Mixes
Organic components should be properly composted and aged with nitrogen and iron additions. While blending composted components with sand, ash or other products, Handreck (1991a) has shown that 1 to 1.5 kg of iron per cubic metre will keep iron inefficient plants well supplied in the long term (1-2 years) and that addition of chelated iron forms can be as low as 200 g/m but will not last as long. For a mix based on equal parts sand, pine bark and sawdust, 4 kg/cubic metre of low P Osmocote (or no P Nutricote for the most sensitive plants), and 1 kg/m (1 gram per litre of mix) of iron sulphate should provide a reasonable result even with P susceptible species. Those plants liking more P (many of the eucalypts and other natives will respond to high P levels) can be supplied with extra P in a liquid feed or topdressing later.
References
Goodwin, P.B. (1981) "Nitrogen, Phosphorus, Potassium and Iron Nutrition of Australian Native Plants". Unpublished Paper, Dept.of Agronomy and Horticultural Science, University of Sydney.
Nichols, D.G. (1988) "Nutrition and Fertiliser Materials". In: Proc. Seminar on Potting Mixes, Artarmon, Aust.Inst.Hort. pp16-30.
Handreck, K.A. (1991a) "Iron Can Partly Prevent Phosphorus Toxicity". Aust.Hort, June-July 1991, Rural Press Vic. pp24-27.
Handreck, K.A. (1991b) "Effective Iron Sources for Iron-Inefficient Plants". Aust.Hort, July 1991, Rural Press Vic. pp26-28.
Jenny, H. (1980) "The Soil Resource; Chap.3, Behaviour of Ions in Soils and Plant Responses. Section F: Aspects of Plant Nutrition in Soils" pg.82. Springer-Verlag, New York Inc.
This article is a reproduction of a paper presented by Simon at the SGAP 17th Biennial Seminar, Robert Menzies College, Sydney, 27 September to 1 October 1993. Simon is the Director/Proprietor of Sydney Environmental and Soil Laboratory Pty Ltd which specialises in analysis and consulting to horticulture, agriculture, landscape architecture, land rehabilitation industries and environmental pollution consultants.
 [Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]
[Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]
The Society for Growing Australian Plants
|