|
[Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]

Understanding Soils and Nutrients
Part 2 - Does Organic Matter?
Jim Barrow
Does organic matter?
Most gardeners are nuts about organic matter. Indeed "organic matter" was once the name of a Perth radio program about gardening. So what is special about organic matter?
You want organic matter to do two contrasting things. On the one hand you want it to persist in soil so that it makes a sandy soil hold more water and makes clayey soil more friable. On the other hand, you don't want it to persist - you want it to break down to supply nutrients to plants, especially nitrogen. So contrasting kinds of organic matter can both be good - but for opposite reasons and serve different functions. This means that there are two properties that matter: the resistance to decomposition and the amount of nutrient released as it decomposes. Before looking at the various kinds of organic matter, we need to consider the effects of organic matter on pH.
There can be two effects of organic matter on pH - direct and indirect. The direct effect occurs if the organic matter itself is alkaline or acid. For example, sheep dung often contains a small amount of free lime and is therefore alkaline. This is because of the composition of the herbage the sheep eat and because of the way they split their excretion between the dung and the urine. However, this is a small effect. By far the greatest effect on pH occurs from release of mineral nitrogen.
To explain this, we need to first remember that there are two main kinds of mineral nitrogen in soil. One is ammonium ("ammonia" when it is a gas). This is exactly the chemical you used to buy in cloudy ammonia or which you can sometimes smell near decomposing organic matter. The other is nitrate - which you can buy as, for example, calcium nitrate.
When organic matter breaks down, some of the nitrogen is released as ammonium. There are microbes in soil which make their living (that is, they get energy) from changing the ammonium into nitrate. How does this affect pH? The ammonium release always makes the soil alkaline. How big this effect is depends on how much is released and on the kind of soil. With very sandy soils, and rich sources of nitrogen, the pH can go up to 9 or even higher. So you can get an effect which is bad for some plants. The irony is that the microbes that are supposed to change that ammonium into nitrate don't really like very high pH so the ammonia can persist for some time. Eventually however, the ammonium does get changed - and that has the opposite effect on pH. It makes the soil acid. For this reason, and for other reasons connected with leaching of nutrients from soil, the long-term effect of adding nitrogen as organic matter is to acidify. So if someone asks whether a particular organic material is acid or alkaline, the only correct answer is the awkward one of "it depends".
 
All the organic matter in your soil, and all that you might add to your soil comes ultimately from plants. Some such as lawn clippings you might add directly or as a mulch. Others you might compost and still others might have passed through a sheep, a cow or a horse. The same broad rules apply to all. One of the rules is concerned with the rate of breakdown. The softer and sappier it is, the quicker it will break down. However, the rate of further breakdown decreases with time. So if the organic matter is already partially decomposed, either in a compost bin or in an animal's gut, further decomposition is slower. This means that if you want organic matter to persist in soil, then the more it has already broken down, the better it will be. If the breakdown has been pretty thorough, say in a compost bin or sheep's or a cow's rumen, then the organic matter has a good soil conditioning effect.
The other rule is concerned with the release of nitrogen. The more nitrogen it contains, the more will be released. For any given kind of organic matter, whether plant material or animal manure, there is a simple relation between nitrogen content and the amount released. Very similar rules apply to the organic sulphur and phosphorus content though these are often not as important.
What about the other nutrients? That depends on the physiology and anatomy of the animal, on the kind of feed they are fed, and on the way the manure is collected. Chooks are perhaps the simplest. These days they are fed a rich diet high in nutrients. Furthermore, their anatomy produces no separation into dung and urine - it all comes out together. So chook manure is high in nutrients but of little value as soil conditioner. Because of its high nitrogen content it can make the soil very alkaline at first if it is applied too heavily - so go easy with it.
Sheep and cattle have a much less rich diet. (Would you believe, chook manure is so rich it can be used as a diet supplement for cattle!). They also have some quirks in their physiology. They don't excrete phosphate in their urine (as we do) but in their faeces. And it is there in a very useful form. It is very finely divided but fairly insoluble. This means it is not prone to leaching loss - which is an advantage on many of our soils. But it is nevertheless readily taken up by plants provided they can get their roots into it. So if it sits on the surface it is not a good source of phosphate but it is very good if it is buried - either by us or by worms. However, there is not much potassium in sheep or cattle dung. "Manure" on the other hand, might contain potassium if it is collected in such a way that the urine has been mixed in with the dung - perhaps this is sometimes the case for sheep manure collected from under a shearing shed.
Horses have a different digestive system with quicker through-put, so horse manure is not quite so far down the decomposition track as sheep's or cow's. So it is going to decompose quite a bit more in the soil. It can also have the disadvantage that horses get treated for worms rather more often than other livestock and residues from the treatments are not very good for earth worms.
|
| "It is, after all, organic matter coatings on the sand grains that make our soils "non-wetting" or water repellent." |
|
Is organic matter always good? No, not always. Although we talk of beneficial effects in sandy soil due to better water holding, the effects can be quite the opposite. It is, after all, organic matter coatings on the sand grains that make our soils "non-wetting" or water repellent. This is a very common problem with lawns which, of course have a lot of organic matter in the soil. It is more often the cause of dead patches than the dreaded lawn beetles - which usually get the blame and the treatment. Nor are our native plants exempt. Those precious specimens you planted last winter and are still watering occasionally may not be thriving because the soil near their roots is not getting wet. Fortunately the commercial soil wetting agents are very effective and well worthwhile using in your garden.
Why re-apply fertilizer?
Here is yet another silly question. Why do we re-apply fertilizer? After all, if we have fixed up deficiencies by applying the perfect dose of nutrients, why isn't that perfection maintained?
The answer to that question is, of course, "it depends". It depends on what you are growing, where you are growing it, what you do with it, and on the nutrient you are talking about. To be more specific, there are five things that can happen to a particular nutrient to make it less effective than it was when you first applied it.
|
| "....there are five things that can happen to a particular nutrient to make it less effective than it was when you first applied it." |
|
It can be removed in some product; it can be incorporated into soil organic matter; or in living plant tissue; it can be lost in leachate; and/or it can keep on slowly reacting with the soil. Let's deal with these one by one.
Losses in products
If you are a farmer, the whole idea is to produce something to "export" from the farm. This of course takes nutrients with it. For example, each tonne of grain removes 2 - 3 kg of phosphorus. In many home gardens, the main product exported is lawn clippings. Nice green juicy lawn clippings can have about 4% nitrogen (N), 0.3 - 0.4% phosphorus (P)
and 2% potassium (K) all expressed on a dry weight. It is no coincidence that the NPK content of lawn fertilizer is in about these proportions.
Suppose you have a lawn that is 10m by 10m. You could easily produce 50 kg of clippings in a year. That's about the NPK in 16 kg of lawn fertilizer: a lot of nutrients. If you have applied more fertilizer, you will grow more lawn with more nutrients.
There is an important message here. Lawn clippings are a darn good source of nutrients. Why throw them away? Just beware that the potassium is present in the sap of cells. As soon as the cell dies, the potassium can be leached out. So if you over-water compost you could lose the potassium. The nitrogen, phosphorus and sulphur content is going to mineralise more slowly and so the decomposing clippings act as a steady source of supply.
Soil organic matter
We have become so steeped in the equation: organic matter equals good, that we may forget that its build-up has a cost. It ties up nutrients - especially nitrogen, phosphorus and sulphur. Again this has been studied for agriculture and we know the amounts tied up as a result of growing pastures - for at least for some environments. The farmer has a choice: he can cash in this capital by growing a crop. In the home garden, our lawns are the equivalent of pastures but few of us are prepared to rotate our crops and plough up lawns. Rather, many people "de-thatch" the lawn. That is, remove organic matter. Again, this is potentially valuable stuff containing expensive nutrients. It could be used as mulch.
What about your native garden? Do you simply drop prunings and let fallen leaves accumulate? Fine. But the build up of a mulch of organic matter does tie up nutrients too. Not as much as in a lawn, but you will have applied far less fertiliser so it can be relatively important.
Plant tissue
Plants do grow and get bigger, slow though that may seem. Indeed in a native ecosystem a high proportion of the available nutrients can be in the living plants, and they don't like letting them go either. We don't have any numbers for your native garden. Just be aware that if your plants are growing they are locking up nutrients.
Leaching
For many parts of the world a simple model describes nutrient leaching: "it scarcely happens".If you have any kind of half-decent soil, most of the nutrient atoms sit on, or even inside, the soil particles rather than in the soil solution. If water moves through the soil it displaces the soil solution but carries little nutrient with it. Further if you have a nice friendly climate, there may be few periods in which rainfall greatly exceeds evaporation so there is little movement of water through the soil.
Alas, poor Perth. Neither of these conditions apply. Many of us have gardens on sands which don't react much with many nutrients and our normal winter rainfall is intense and occurs when there is little evaporation. A lot of water moves through the soil in winter and takes nutrients with it. Where does that water go? It becomes ground water of course. So if you put in a bore you might argue that you are recycling nutrients as well as water!
If we water sensibly in summer, and all Wildflower Society members would, there should be little movement of water to deep layers and so little possibility of leaching. The limited water we apply would all be used by plants rather than percolating into the soil.
Two more points on leaching.One is that native plants are designed to limit any loss of precious nutrients. They have a dense surface root system which is active in winter and tries to catch all nutrients before they can get away. And they have a deep root system which is active in summer to pull up water from the ground water. The other point is that not all nutrients are equal in this regard. Some are held much more tightly by the soil and are less prone to loss. More on that later.
Slow reaction
Suppose you apply some phosphate to a soil. You don't grow plants, you don't let leaching occur, you just keep the soil moist. What happens to that phosphate? It keeps on reacting slowly with the soil. It becomes harder and harder for plants to get it. The fertiliser becomes less effective. We are not talking about a small effect here; as you can see from an experiment I did many years ago, it is a large and important effect.
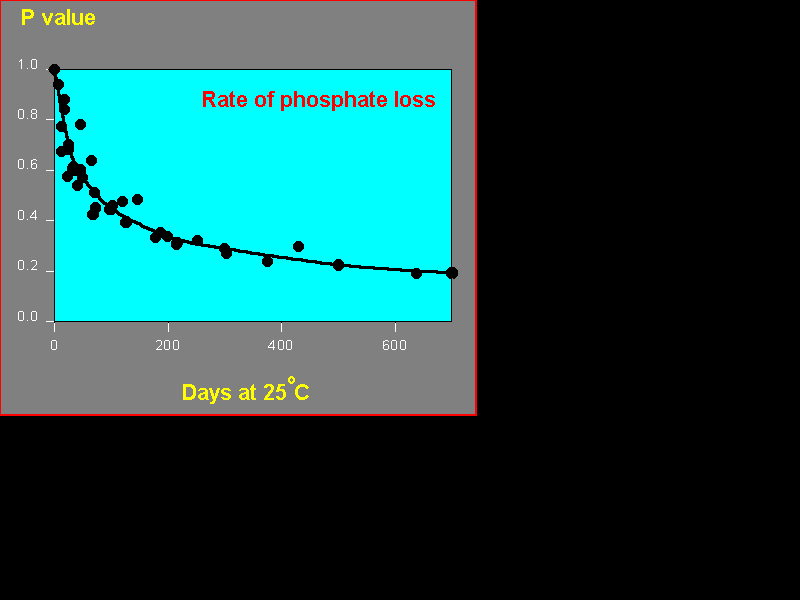
Nor is the effect confined to phosphate. It happens for all of the nutrients that react strongly with soil: phosphate, molybdate, copper, zinc (and animal nutrients such as cobalt and selenite). It even happens if the soil is dry - though more slowly - and it goes much faster if the temperature is increased. How come? What is the mechanism? We are now pretty sure that the nutrients first react with the surface of soil particles - they are adsorbed. But then they slowly diffuse into faults in the structure of the particles. It is "solid state" diffusion. That is why it can happen even if the soil is dry. It goes faster at higher temperature because that helps the atoms to jump about. And the further they go in, the slower they come out again so plants find it harder to get hold of them.
In summary
You do need to re-apply fertilizers. Even native gardens benefit from some fertilizer. But apply little and often if it is soluble fertilizer, or use slowly soluble forms.
Jim Barrow is a member of the Wildflower Society of Western Australia and is a "semi-retired" CSIRO soil scientist.
This series of articles was first published in the Newsletter of the Wildflower Society of Western Australia during 1998-1999. Part 1 of the series appeared in the March 1999 issue of "Australian Plants online". The third and final part will be published in the September 1999 issue

[Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]
Australian Plants online - June 1999
Association of Societies for Growing Australian Plants
|