|
[Front Page] [Features] [Departments] [Society Home] [Subscribe]

Short Cuts
Short items of interest about Australian plants selected from the many newsletters and journals published by member Societies of ASGAP.......
Short Cuts in this issue:
 Plants' Adaptation to Drought Plants' Adaptation to Drought- Can we apply plant's coping mechanisms under garden conditions?
 Growing Epacris in Containers Growing Epacris in Containers- Australian heaths are ideal plants for growing in pots.
 Germination of Epacrls Seeds using Plant-derived Smoke, Darkness and Heat Germination of Epacrls Seeds using Plant-derived Smoke, Darkness and Heat- Although smoke treatment may improve germination of Epacris seed, other factors should also be considered.
 Olearia: The Daisy Bush Olearia: The Daisy Bush- Successful cultivation may depend on the origin of the particular species
 Grafting Eremophila Grafting Eremophila- A modified wedge graft gives excellent results
 The Red Wattle The Red Wattle- The story behind the red-flowered wattle involved as much good fortune as skilled propagation

Plants' Adaptation to Drought
Plants have developed a whole range of mechanisms to cope with dry conditions. Margaret Guenzel wonders whether we should consider theses adaptations when growing plants in cultivation.
Some time ago, "The Age" newspaper carried an article by Tom Neales which dealt with plants' adaptation to drought. The most interesting point was that recent research has discovered that when the soil around a plant's roots begins to dry out, the roots send hormonal signals to the leaves, which results in the partial closure of the leaves' stomata. A multi perforated surface (in this case the leaf with its stomata) loses as much water vapour to the air as a water surface completely open to the air.
For a plant to reduce water loss is not just a matter of closing its stomata because the main purpose of the stomata is gas exchange for photosynthesis which is only possible in sunlight. A second reason why stomata cannot just be closed up during the daytime is that complete closure would result in overheating of the leaf, because evaporation also provides cooling. So plants have adapted in a number of ways to minimise water loss. Here are just some examples. The mechanisms are often quite complex.
- Plants can grow lots of surface hairs which trap water molecules close to the leaf surface thus minimising further evaporation.
- Some plants deliberately "wilt" i.e. they adjust the water saturation inside the leaf which may only be 50% to reach an equilibrium (I found this in some of my thomasias). Some succulents and cacti almost stop photosynthesing and close up shop.
- Many eucalypts position their leaves parallel to the sunrays to expose as little as possible of their surfaces to the sun.
- Some plants reduce the size of their leaves during the hot summer months i.e. the leaves produced later in Spring are smaller than their earlier ones.
- Some eucalypts photosynthesise only during the cooler morning hours.
Some plants sacrifice surplus growth and die back a bit to save themselves, others drop a lot of their leaves during drought and "make do" with a minimum....I am sure you all know of a few more examples.
To come back to the article in "The Age", there are trials in progress in South Australia in vineyards. Only one side of the root system of the plants is watered so some of the roots will still signal to the leaves that a drought is coming and evaporation must be reduced. This is supposed to save water but not affect yields. It is called PRD or partial root drying.
Maybe it would work for us. I have often wondered whether we don't nullify some of these mechanisms of our plants by watering them too often and so make them more likely to die from heat stress. Maybe we should.group our plants better, restrict ferns and rainforest plants to smaller spots and plant more dry area plants together and leave them be.
From from the newsletter of the Foothills Group of the Australian Plants Society (Victoria).
[ Return to Index
]

Growing Epacris in Containers
Many people are restricted to growing plants in containers because of limited space or other reasons. Gwen Elliot explains why Epacris species are excellent subjects for containers.
Why Grow Epacris in Containers?
Container cultivation can be useful for a variety of reasons:
- We may not have the space for a regular garden.
- We may find container-gardening physically easier than in ground planting.
- Tubs or hanging baskets can be useful in paved courtyards or similar areas.
- Container cultivation allows us to bring a plant to a focal position when in full bloom, and to change plants around as each reaches its peak.
- Growing plants in containers allows us to vary the potting mix for each plant. This is particularly useful if our garden soil is not suited to certain plants we wish to grow.
- We are able to also adjust watering, fertilising etc., to suit the needs of each individual plant when we are growing them in containers.
- We can move containers from season to season to suit the needs of the plants being grown, ie. in a sunny position in winter, but with more shade in the hot summer, or in a protected position during winter in areas of severe frost.
Choosing the Right Container
Epacris can be grown successfully in containers made of terracotta, glazed or unglazed ceramics, plastic, timber or metal. If timber is used it should be of a durable material which will not rot down quickly, but materials such as treated pine should be avoided as the chemicals are likely to adversely affect the plants. Metal containers are occasionally seen, but these should not be used in hot sunny situations as heat is transferred through to the potting mix and the tender roots of plants such as Epacris can suffer burning. Metal containers should also be avoided in areas of heavy frosts.
Hanging baskets can be made of the above materials, or are also obtainable made from wire frames with linings of fibre, recycled wool, or recycled car tyres. All are suitable for use with Epacris.
Pots with a diameter and depth of 25-30 cm or more are usually very suitable for the cultivation of Epacris plants for several years.
All containers should have adequate holes to allow the potting mixture to drain freely.
 |
 |
Epacris impressa is one of the best known members of the genus. The pink form (left) is Victoria's floral emblem but white flowered forms (right) are also common.
Select the thumbnail image or highlighted name for a higher resolution image (39k and 41k).
|
Potting Mixes
A good quality standard potting mix is recommended for the cultivation of Epacris in containers. A potting mix without fertiliser etc. already added allows the user to select any appropriate additives for the specific plants being grown. It is better to avoid cheap potting mixes, without the Standards Association ticks of approval.
Fertilisers
The use of a low phosphorus, slow release fertiliser at the time of planting will assist the initial growth of Epacris plants as they become established in the container. Subsequently a light application of similar fertiliser each spring is usually sufficient to ensure ongoing health and vigour of container-grown plants. Liquid seaweed can also be used with success on Epacris plants in containers.
Further input from growers on fertilisers they have found to be useful would be very welcome.
Watering
Containers need to be watered regularly to ensure that the mix is kept moist but not waterlogged. Good drainage holes in pots are important. Water retaining granules or crystals can be added to the potting mix if desired and self watering containers can also be used with good success.
From the March 2001 issue of the newsletter of ASGAP's Epacris Study Group. Gwen is the Study Group leader.
[ Return to Index
]

Germination of Epacrls Seeds using Plant-derived Smoke, Darkness and Heat
There has been considerable research into the use of smoke as an aid to seed germination but other factors may also be important....as Craig Gilmour explains.....
A wide range of native plants with previously sporadic or poor seed germination rates have been shown to have seed germination promoted by smoke derived from the combustion of plant material.
Several species from the Epacridaceae family have shown a positive response to plant derived smoke and/ or smoke products.
In my own experiments, Epacris tasmanica seeds were subjected to a combination of heat, dark, and smoke concentration treatments (16 treatments in all including the controls), to determine which treatments gave rise to the highest percentage and highest rates of germination.
The best treatment was then applied to three other Tasmanian Epacris species, (E.obtusifolia, E.lanuginosa and E.apsleyensis), along with the mainland species E.purpurascens, for a comparison of germination rates and percentages.
The E.tasmanica treatments were as shown in the following table.
- Dark
- Smoke
- Heat
- Dark, Smoke
- Dark, Heat
- Smoke, Heat
- Dark, Smoke, Heat
- 5% Smoke, Heat
|
- 5% Smoke
- 5% Smoke, Dark
- 5% Smoke, Dark, Heat
- 10% Smoke, Heat
- 10% Smoke
- 10% Smoke, Dark
- 10% Smoke, Heat, Dark
- Control
|
All seeds were germinated on filter papers in petri dishes, with 6 replicates of 50 seeds used for each treatment.
Treatments
Smoke Smoke solutions were generated by burning both green and dry native vegetation and then using a vacuum cleaner to bubble smoke through two litre containers of distilled water for half an hour. Both the initial solution generated, and 5% and 10% dilutions of the original smoke solution were used for the treatments. Smoke solutions were applied to the seeds for the first 4 waterings after which all seeds were watered with distilled water.
Heat Seeds were placed in aluminium foil envelopes and pre-heated in a convection oven at 90oC for 10 minutes prior to smoke or distilled water treatments.
Dark Dark treatments were placed in a light proof cardboard box in an incubation room set at 25oC. All other treatments were kept in the same incubation room but exposed to a 16 hour photoperiod.
Control The controls, along with all other treatments, not including smoke, were watered with distilled water.
Epacris tasmanica
In one month 46% of seeds germinated in the 5% Smoke, Heat, Dark treatment, with the majority of the germination occurring within the first 2 weeks. Whereas the Controls took a month before beginning to germinate and the germination was sporadic with only 3% of seeds germinating.
The second highest germination percentage was observed in both the 10% Smoke, Dark, Heat treatment and the 10% Smoke, Heat treatment. Both treatments had 29% germination.
However, all the other treatments had germination percentages of less than 20%, with the majority, such as the 5% and 10% Smoke treatments having germination percentages of less than 10%.
Only 4% of the seeds in the full strength smoke solutions germinated, mostly towards the end of the second month of the experiment perhaps due to continuous watering gradually diluting the smoke solution to a level where it did not inhibit germination.
The 5% Smoke, Dark, Heat treatment which was the best treatment was applied to the other Epacris species:
- Epacris purpurascens showed the greatest germination percentage with 67%, of seeds germinating within 2 weeks of the experiment being set up. In comparison only 1% of the Controls germinated.
- Epacris apsleyensis - 63% of the seeds germinated within 2 weeks, whereas only 4% of the Controls germinated.
- Epacris obtusifolia - 34% of the seeds germinated within 2 weeks, whereas only 1% of the Controls germinated.
- Epacris lanuginosa - 31% of the seeds germinated within 1 week of the experiment being setup, whereas less than 1% of the Controls germinated.
It can be seen that all five Epacris species responded positively to the combination of 5% Smoke, Dark, Heat treatment, and all five species had only low levels of germination in the light in the absence of heat and diluted smoke solutions. However, smoke solutions by themselves did not greatly increase the percentage of seeds which germinated when compared with the number of seed which germinated in the controls. The germination responses suggest that all five species probably have fire related seed dormancy adaptations, but they also possess relatively small non-dormant seed fractions.
From the September 2001 issue of the newsletter of ASGAP's Epacris Study Group.
[ Return to Index
]

Olearia: The Daisy Bush
The wide geographical range of Olearia species suggests that careful thought should be given to their placement in the garden. Jenny Rejske elaborates....
Olearias grow in the mountains as well as along the coast, in hot dry areas and in cool damp forests, and show off their charms and their flower-heads on low spreading shrubs or quite tall trees. So with their wide range of habit and habitat they fall into three large groups:
- Dry inland
- Temperate and coastal
- Mountain
Near the coast and in the forests of Victoria olearias do well, enjoying the cool, temperate climate and good rainfall. Olearia ramulosa, O.asterotricha, O.glandulosa and O.erubescens can be found growing in these areas, whilst O.glutinosa and O.axillaris prefer the coastal sands and sandstone.
Olearias of the arid region flower in profusion from late winter through spring. These include white flowering O.pimelioides, O.muelleri, O.microphylla and blue mauves of O.rudis and O.ciliata. O.pannosa has large, showy, white heads and O.magniflora is an insignificant, spindly shrub until it comes into flower with lilac coloured daisies, 5 cm across, covering the bush.
Olearias of the mountain areas are usually quite large bushes, tall and sturdy, with big leaves. This group includes O.lirata, O.phloqopappa, O.argophylla and O.megalophylla.
So when you stand with an Olearia species in hand ready to plant in the garden, give some thought to which area it would have originated from and plant accordingly:
- Dry inland - full sun
- Coast and temperate - sun/dappled shade
- Mountain - shade
 |
|
Olearias can be found in a wide range ofd colours.
Olearia persoonioides (top) and Olearia tomentosa (bottom).
Select the thumbnail image or highlighted name for a higher resolution image (40k and 24k). Photos: Brian Walters and Australian Daisy Study Group
|
 |
Propagation
- Seed
- Seed does not germinate well.
- When pricked out a large percentage of seedlings die off.
- Do not sow too thickly and prick out when small.
- When established grow on well.
- When planted in the garden they often die and even established plants die in pots.
- Cuttings
- Cuttings root easily.
- There is a large percentage strike.
- There are no problems in potting on.
- There has been some trouble with furry species (e.g. O.asterotricha) due to stem rot and fungal attack.
- Plants grown from cuttings also die in the garden and established plants die in pots.
From the newsletter of ASGAP's Australian Daisy Study Group, November 1988.
[ Return to Index
]

Grafting Eremophila
Colin Jennings, leader of ASGAP's Eremophila Study Group, reports on the research carried out by Group member, Ray Isaacson
Ray Isaacson has had enormous success with his grafting, using Myoporum acuminatum and M.montanum as the stock plants. He has persisted and now feels that he has made a significant breakthrough, despite having said on a number of occasions that he "was not going to do any more".
Ray has been an advocate of using very soft tissue as the scion material, grafting this onto young, actively growing stock. He has tried various techniques over the years, ranging from the use of Eremophila maculata as the stock, to the use of the prostrate growing Myoporum parvifolium, as well as using much older stock and larger, more mature cuttings from which to take the scion material.
The wedge graft has been the most popular for use with eremophilas, with the stock being cut completely through to the depth required for the wedge-shaped scion to be inserted to its full depth. The problem with this has been that often the scion and stock material do not match up and the marrying of camblum tissue has been rather 'hit and miss'. This has been overcome to some extent by ensuring that at least one of the prepared edges matches up with the edge of the stock.
Binding the scion to the stock to ensure a good union has been achieved by the use of a film used in laboratories to seal petri dishes etc. This film 'breathes' and also stretches, allowing for expansion of the tissue as union occurs and tissue growth takes place. This has been found more satisfactory than using the grafting tape used in the horticulture industry for tying grafts on trees etc.
Ray has now developed a modification of the wedge graft technique.
He still uses very young scion material and young, fresh growth on the rooted stock plants. The major differences are:
- The stock is cut off just below the second pair of leaves above ground level. This gives a good length of stem to work with and allows sufficient leaves to ensure photosynthesis is continuing. It also ensures that there is less water loss through transpiration.
- The scion is prepared by making a wedge shape, but one of the sides is cut away to leave only one surface with its outer surface still intact. The wedge is thus halved.
- The incision into the stock is only made part way into the stem and vertically as shown in the diagram below.
- It is important to only cut as deeply into the stock as is necessary to accommodate the prepared scion.
- The scion is then placed into the prepared slot and positioned so that the outer surfaces of the scion and stock are aligned.
- Parafilm or Nescofilm is then wrapped around the scion and stock to ensure that the graft is fully protected both above and below the union.
- A small zip-lock plastic bag is then placed over the graft and the plant placed under protection until the union has been made. This can take as little as ten days - different species seem to vary.
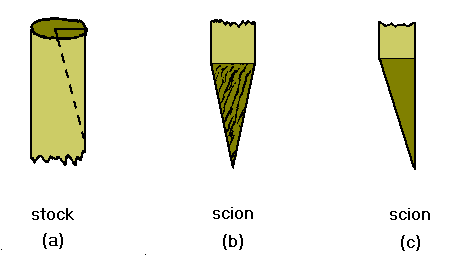 |
| Shave scion to form a wedge, then turn through 90o and shave to suit incision made in the stock. |
The best time to carry this out is in January/February. Ray says that any earlier or later can be successful. however, he has achieved excellent, in fact 100%, results with some species, by doing them at this time. Growth is active in both the stock and the eremophilas being grafted.
Grafting has been found to be most successful in establishing newly discovered or hard to strike species. Without the work in this field it would have been almost impossible to have some of the very desirable species in cultivation which we now see. It is thanks to Ray and others who have persevered with this technique that we can now see such species as E.mirabili, E.'enata', E.spectabilis and E. 'Iucida' 'in our collections. Whilst some of these could have been introduced via cuttings, it would have taken a lot longer and with fewer successes.
From the August 2001 issue of the newsletter of ASGAP's Eremophila Study Group.
[ Return to Index
]

The Red Wattle
Acacia leprosa "Scarlet Blaze" - a newly discovered wattle is Victoria's Federation floral emblem
The "Scarlet Blaze" story began in 1995, when a single red-flowered Cinnamon Wattle was discovered by bushwalkers in state forest north east of Melbourne. A number of cuttings were apparently taken, two of which arrived at Melbourne's Royal Botanic Gardens.
 |
|
| Select the thumbnail image or highlighted name for a higher resolution image (34k). |
|
Michael McNabb, the Melbourne Gardens Nursery Co-ordinator, has been involved since 1996 in the Gardens' efforts to propagate the original cuttings. Michael says that different propagating media and hormone supplements were tried, with varying degrees of success.
"Once we found the right combination, the plant has proved relatively easy to propagate, with about a 50% strike rate. We also discovered that propagation by cutting is the only reliable method, as the flowers revert to their common yellow colour if the plant is propagated from seed."
"The parent plant in the bush has since died", says Richard Barley, Divisional Director of the Melbourne Gardens. "As far as we know, none of the other cuttings survived, so the results we've managed to achieve here have been very significant in terms of conserving a truly unique plant."
The Gardens has lodged an application for Plant Breeders Rights, and contracted Plant Growers Australia, a large wholesale producer, to grow the plant for the commercial market and to co-ordinate national sales and growing trials.
From a Media Release by the Royal Botanic Gardens Melbourne
[ Return to Index
]

[Front Page] [Features] [Departments] [Society Home] [Subscribe]
Australian Plants online - December 2001
Association of Societies for Growing Australian Plants
|