|
[Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]

Short Cuts
Readers are invited to submit short items of interest about Australian plants to be included here. If submitting non-original material (eg newspaper or magazine cuttings), please also advise if the author has given permission to republish and, if not, please provide a contact address so that permission can be sought.
Short Cuts in this issue:
 Pasteurised Cutting/Potting Mix Pasteurised Cutting/Potting Mix
- A new use for household "white goods"
 Pruning to Rejuvenate Pruning to Rejuvenate
- When in doubt....cut it back!
 The White Waratah The White Waratah
- The white form of Telopea speciosissima has links to aboriginal legends
 The Blueberry Ash The Blueberry Ash
- A tropical species which adapts well to colder areas...in this case, Tasmania
 Vegetative Propagation of Ferns Vegetative Propagation of Ferns
- Many ferns can be propagated simply by vegetative means
 Tripping Triggers! Tripping Triggers!
- Discover the bizarre pollination methods employed by trigger plants

Pasteurised Cutting/Potting Mix
Improve your plant propagation success using this simple disinfection technique
Where do you get pasteurised cutting/potting mix? You make it! The same treatment applies to the sterilisation of a mix to receive cuttings, as for a mix to take rooted plants after they have struck.
- Add water to the cutting/potting mix until it is moist enough to be used for potting on cuttings or plants.
- Fill a 4 litre ice cream container with the moist mix. Place the container, without a lid, in a microwave oven.
- Zap it at full power (about 700 watts) for seven minutes. Use a longer time if your microwave's top setting is below 700 watts (your instruction book will state the power).
- Remove the container from the oven and immediately place it in an "Esky" or similar insulated box. Leave it for 30 to 60 minutes. The aim is to ensure that the mix remains above 60 degrees C for at least 30 minutes. This treatment does not kill all microbes but it does kill those pathogenic microbes that cause damping off and other root rot diseases. It also kills mycorrhizal fungi and rhizobium bacteria.
The mix is ready to use at the end of this storage period.
Placing a lid on the container after the treatment will lessen contamination by damping off pathogens. But beware! Be a good housekeeper! Keep your pots, cutting frame and shade house free from contamination or you will have wasted your time preparing the pasteurised mix.
Adapted from the August 1995 issue of the Journal of the South Australian Region of SGAP.
[ Return to Index ]

Pruning to Rejuvenate
Connie Spencer shows that it's never too late to bring recalcitrant plants into line.
Well, almost.....
My front garden in Alice Springs consists of a selection of Australian trees and shrubs which have been in for about seven years and, apart from pulling out annual weeds and controlling any couch grass that comes up, I have very much left plants to grow as they will over the years. Most have grown well, are healthy and are providing a screen for our house and pool.
However, some plants were past their best and had developed a leggy and woody appearance with foliage only on the ends of the branches. Others had been attacked by one pest or another.
I have four Alyogyne hakeifolia (a native hibiscus from the drier parts of South Australia and Western Australia) which are excellent screen plants. They are dense shrubs with fine, bright green foliage and are absolutely magnificent in late spring when covered in cream hibiscus-like flowers. They had been attacked by a scale and I had left them to fight it themselves. All the greenery was now on the ends of the branches with nothing in the middle so, not only did they look unsightly but certainly were not providing a screen anymore.
Cassia sturtii had become straggly and woody. Each year it produced fewer flowers and certainly was not the spring showplace that it had once been.
Many of my eremophilas (or Emu Bushes as they are commonly called) had been attacked by a webbing caterpillar which is a common complaint with eremophilas. This made them most unattractive. Some had developed a woody appearance with foliage only on the ends of branches as with the Alyogyne.
Something had to be done but before I got rid of them altogether, I decided on drastic pruning with the hope that the shrubs would regenerate from the base. They certainly were not an attraction in the garden and were not doing the job they were intended to do, so I had nothing to lose. Although it is considered advisable to prune when the sap flow is at its lowest (early to mid-winter), I decided to prune in late winter after any likelihood of frost was over as the soft new growth which I was hoping for would be very susceptible to frost.
With one large Alyogyne, I cut it right down to three or four stumps about 30cm long. The stumps were about 10cm in diameter and there was no greenery left at all. This is against pruning principles but there was a seedling not far away so it did not matter if I lost the large one. Within a couple of weeks the stumps were covered with new shoots. It is now a dense, rounded shrub about 1m x 1.5m.
The other not so large specimens of Alyogyne, I pruned so that each branch has some greenery left on the ends, and each week as new growth appeared further down the branches, I would prune back closer to the main trunks and to the shape I wanted. I did this for about a month and eventually they were also covered with fresh green growth from the main trunks out.
I pruned back the Cassia to about 10cm above ground level. There were three or four new stems shooting from the base of the plant. The branches that were left sticking out of the ground did not re-shoot but more new stems grew from the base. It is now a healthy, rejuvenated-looking shrub.

Many callistemons and melaleucas respond to the heavy pruning technique described. This Callistemon "Captain Cook" shows good, vigorous regrowth after being cut back to near ground level. Select the thumbnail image or plant name for a higher resolution image (45k).
With the eremophilas I found that some could be pruned drastically to regenerate growth but others couldn't. Eremophila maculata and many of its various forms seems to be able to take very hard pruning. Not so with E.macdonnellii. E.glabra responded quickly to severe pruning but gradually the new shoots shrivelled up and the plants died. I pruned these much earlier - June, I think, so perhaps the new shoots needed warmer weather to continue growing.
To a certain extent, much of this pruning could have been avoided if I had been tip-pruning the plants from the beginning and removing excessive or poor growth as they grew, rather than letting them become unattractive and giving Australian plants a poor name.
From the March 1997 issue of the SGAP South Australian Region's "Journal".
[ Return to Index ]

The White Waratah
Although becoming more widely available, the white waratah is still regarded as a novelty and never fails to attract attention. Its story is told by Susan Heins.
The "Australian Plants" journal of March 1988 listed a few waratah cultivars. There was no mention of the White Waratah, but it exists.
I was staying for a few days with my friend Thistle Stead (Harris) at Wirrimbirra when, one morning, Red Mitchell from the Water Board came excitedly in and said to us "Come with me and I'll show you something very special". He drove us along a small road in the Water Board Reserve at Picton Lakes to a tree he had marked and, from there, he led us into the bush.......and there was a small bush only about 1 metre high of a pure white waratah.
The day before, a Water Board driver, driving along the bush track saw something white and stopped to investigate. He saw a bush with 5 -6 big white flower heads but did not have a clue what it was. He broke one head off and took it with him when he went after work to the Picton pub. That night, by good luck, Red Mitchell was there and he realised immediately what the flower was. He got the driver to show him where he found it and the next morning he came to Wirrimbirra to fetch us. The plant was badly burnt and there was not much new growth on it. There was not much cutting material but we got a few pieces.

The unique white Waratah Telopea speciosissima "Wirrimbirra White" was collected in the early 1970s and first propagated at Wirrimbirra. Select the thumbnail image or plant name for a higher resolution image (32k).
Wirrimbirra produced two flowering plants, both very slow growing. Thistle gave some cuttings to experienced growers with the request that no plants be sold. The Canberra Botanic Gardens got some and soon produced 2 beautiful plants, about 6 feet tall and with many flowers.
The Picton Lakes plant is not the only white waratah I have seen. On one of my trips with Thistle we drove through Colo Vale. There in front of an old cottage was a magnificent waratah bush 6 feet tall covered with pure white flowers. We asked the old woman living there to let us take some cuttings but she said "Nobody will touch my white waratah". Two years later we passed there and got the same answer. 2-3 years later Thistle drove through Colo Vale and there was no more white waratah. It had died and nobody was ever allowed to take any cuttings of it.
The white waratah was known by the early aborigines. In the Dreamtime it was the only colour form known. Among the stories and legends handed down is the following:
"Searching for her lost mate a Wonga Pigeon flew high above the tree tops forgetting about her enemy, the dreaded hawk. She was attacked and his talons inflicted severe wounds. But the little pigeon freed herself and, with blood dripping from her wounds, she flew down among the white waratahs. Her blood dripped on them turning the white flowers red. At last reaching her mate, the pigeon shed the last of her blood and since that time all waratahs have been red." But the legend concludes, "Sometimes, although it is rare, it is still possible to find a white waratah as they were in the Dreamtime".
From the July/August 1988 issue of "Native Plants for New South Wales", the newsletter of the New South Wales Region of SGAP. Susan Heins was a long time member of the Society and held a number of executive positions. She died in 1991.
[ Return to Index ]

The Adaptable Blueberry Ash
The blueberry ash may well be the ideal garden plant for temperate, sub-tropical and tropical gardens. George Wade has found the Tasmanian climate no challenge for it
About 20 years ago I attended a conference in Queensland and, on the weekend, I was taken to Bribie Island. As we drove across the island, my attention was arrested by a beautiful large shrub with racemes of white flowers, which was growing by the roadside. I had never seen it before so I took a sample and showed it to some Queensland friends, who did not know it. I kept it in water for the week of the conference and then brought it home to show my wife. As it was still in good condition I put in some cuttings and one of them struck.
When it was well established, I planted it in the garden where it has done well and is now about 5 metres high. Every year it rewards me with masses of flowers in January, followed by blue berries which persist throughout the year. The mistake I made was to think that because it came from Queensland, it should be placed in the warmest spot in the garden, with full northerly exposure. I note that Wrigley and Fagg ("Australian Native Plants") state that the species prefers some shade and I must admit that the leaves of my plant develop brown spots, which are probably a result of the exposed position.
When my plant flowered I decided to see if I could work out the family to which it belongs, using the key in Dr Curtis' "Student's Flora of Tasmania". In fact it keyed out to both the genus and species and I found that Elaeocarpus reticulatus occurs on King Island and in all eastern mainland states.

The colourful fruits of Elaeocarpus reticulatus are held on the plant for a long period. Select the thumbnail image or plant name for a higher resolution image (30k).
The leaves are lanceolate, about 6 to 10 cm long and have serrated margins and conspicuously reticulate veins. The young leaves are pale green with reddish tints. The flowers are very beautiful with deeply lobed petals about 1 cm long, forming bell shaped flowers somewhat similar to those of Aristotelia peduncularis, which belongs to the same family (Elaeocarpaceae). The fruit is also attractive being a blue drupe about 1 cm long.
When one of my sons was married, his bride asked if I would make the bridal corsage and I decided Elaeocarpus would be very suitable. I was intrigued to learn years later that Wrigley and Fagg state that it is used in bridal bouquets.
I had read that there was a pink flowered form but I had not seen it until I visited Ken Gillander's nursery and found that he had a flowering specimen for sale. It is now in my garden, this time in a shaded position, and it is looking healthy and flowered freely this year.
From the March 1988 issue of the newsletter of the Tasmanian Region of SGAP.
[ Return to Index ]

Vegetative Propagation of Ferns
Ferns are grown by gardeners all over the world. Few people think about propagating their own but, as David Jones demonstrates, some are particularly easy.
Australian ferns are popular subjects for cultivation and propagation techniques are important to alleviate the pressures of indiscriminate collecting from the bush. Ferns are readily propagated by vegetative means; the techniques are generally simple and well within the scope of enthusiasts and commercial nurserypersons.
Terms Used
In the following notes a few terms will be used which may require explanation. Their use is obligatory when dealing with such a specialised subject.
- spore - a tiny vegetative reproductive unit which contains only half of the normal complement of chromosomes and no embryo.
- prothallus (plural; prothalli) - a flat growth resulting from the germination of a spore and bearing the sexual organs.
- archegonium - the structure on the prothallus which produces the female egg.
- antheridium - the structure on the prothallus that produces the male sperms.
- sorus (plural; sori) - the site on the underside of the fronds where the spores are borne.
- sporangia - the mechanism in the sorus for shedding the spores.
Division
Ferns with creeping rhizomes such as Hypolepis, Microsorium, Lastreopsis culcita or Arachniodes, can be readily increased in number by dividing the rhizomes. These can be cut into lengths and those pieces with an active growing shoot or apex can be potted into new mixture.
Dormant divisions which lack an active growing shoot, can be potted together in a well drained mixture, or sphagnum moss, and placed in a warm area or in a propagation bed fitted with bottom heat. They will initiate new growth and can be potted separately when established.
Division is best carried out when the plants are in active growth during the warm months of the year. Some species which don't have creeping rhizomes form multiple crowns which can be divided every 2-3 years (eg. Pteris tremula, Polystichum formosum).
Offsets
Some ferns produce runners which bear new plants near the apex. These can be separated when sufficiently well developed and established as separate plants. Species include Blechnum nudum, B.chambersii, B.fluviatile, Doodia media and D.aspera.
A couple of tee ferns, viz. Dicksonia youngiae and Cyathea rebeccae, produce offsets along the trunks or from underground runners. These can be removed when large enough and established as separate plants, although they may be slow for a while. Offsets on elkhorns (other than Platycerium superbum) can be removed with a sharp knife and mounted independently on a new slab.
Bulbils or Plantlets
A number of ferns produce bulbils or plantlets along the fronds. These usually appear as small buds about 12 months after the fronds develop. As the frond matures, it is forced down by the emergence of younger fronds, and the plantlets enlarge and develop. As the frond nears ground level the plantlets take root and eventually become independent of the parent plant.
This is a useful character for propagating some species. The plantlets can be removed from the fronds before the frond collapses but they should be mature and well developed, otherwise they will collapse. They must also be kept in a humid situation until well established. Species which can be propagated in this manner include Asplenium bulbiferum, A.attenuatum, Polystichum australiense and P.proliferum.
Auricle Cuttings
Species of Angiopteris and Marattia produce fleshy ear-like structures, known as auricles, at the base of the fronds. Old fronds can be cut from the rhizome below the auricles and the frond stem split to give two cuttings each containing an auricle. These can then be planted in a well drained propagating mixture and plantlets will form at the base of each auricle.
This technique is best carried out during warm weather and the results may be hastened by placing the cuttings in a glasshouse or bottom-heat propagation unit. The method is slow but very valuable as these handsome ferns are impossible to raise from spore by conventional techniques.
Layering
A specialised technique useful for species of the genus Lygodium. These ferns produce long climbing stems. A stem section bearing nodes can be induced to form roots and new shoots by pegging down and covering lightly with soil. Once established it can be separated from the parent plant.
From the September 1978 issue of "Native Plants for New South Wales", the newsletter of the NSW Region of SGAP.
David Jones is a professional horticulturist with a keen interest in the Australian flora. He is the author of more than 15 books and is co-author (with Rodger Elliot) of the definitive "Encyclopaedia of Australian Plants".
[ Return to Index ]

Tripping Triggers
Frank Carroll explains the strange reproductive behaviour of Stylidium species and the shock that is in store for any insects which visit the flowers.
This is not the title of the latest wild west movie, but a perfectly innocent pastime indulged in by wildflower lovers who happen to go bushwalking in summer in any area which is home to the genus Stylidium.
This large genus contains the 130 or so Trigger Plants. Look for a perennial herb, from about 5cm to 70cm tall, with one or more spikes of pale pink to deep mauve flowers. (In Western Australia there are also species with red, yellow or white flowers.) Each flower appears to have four petals (actually there are five, but one is very small). To one side, between two of the petals, is a column made by the fusing together of the stamens and the style. It is sensitive to touch, and remains cocked until an insect goes to seek nectar in the centre of the flower, whereupon the trigger flies round and hits the visitor on the back.
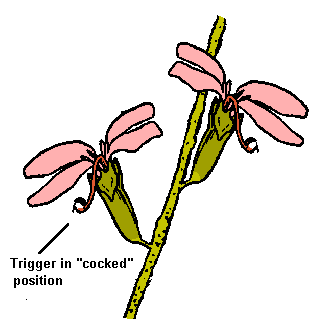 One can deceive the flower by touching it with a thin stem or grass blade; hence our pastime. After some minutes the trigger reverts to the ready position. The stamens develop before the stigma, so that a new flower dusts pollen on the backs of insects. As the flower matures the stamens cease activity and the stigma develops, picking up pollen brought to it from other flowers. Another one of the marvels of nature is that each species of Stylidium has its trigger of a characteristic length and swinging at such an angle that pollen is deposited and collected from its own particular spot on the back of the insect; so that when several species grow close together, hybridization is largely avoided.
One can deceive the flower by touching it with a thin stem or grass blade; hence our pastime. After some minutes the trigger reverts to the ready position. The stamens develop before the stigma, so that a new flower dusts pollen on the backs of insects. As the flower matures the stamens cease activity and the stigma develops, picking up pollen brought to it from other flowers. Another one of the marvels of nature is that each species of Stylidium has its trigger of a characteristic length and swinging at such an angle that pollen is deposited and collected from its own particular spot on the back of the insect; so that when several species grow close together, hybridization is largely avoided.
Trigger Plants occur in south-east Asia, Australia and New Zealand, but Australia is their headquarters. Authorities differ as to the exact number of Australian species, but it is approximately 120: 14 species in Queensland, 7 in New South Wales, 6 in Victoria, 7 in South Australia, at least 1 in Tasmania, 13 in the Northern Territory and 86 (!) in Western Australia.
There are two common growth habits. The first is a clump of long grass-like leaves, with a tall unbranched flower spike rising from its centre. The other is a rosette of rather broad spoon-shaped leaves that rest flat on the ground, and give rise to one or several shorter, many-branched flower spikes resembling the shape of miniature Christmas trees.
The tall, grass-leaf trigger plant, Stylidium graminifolium, with its bright pink or mauve flowers, is a feature of grassy areas of the Blue Mountains and the Kosciusko Plateau (the alpine meadows around The Chalet at Charlotte Pass are aglow with it every January) in New South Wales and of the Tasmanian central highlands. In other parts of eastern Australia they are not so conspicuous, though they are not rare.
In February of 1979 or 1980 I was delighted to discover by accident a colony of trigger plants at the top of Hervey's Range, close to the main road, west of Piper's Lookout, at the edge of an open forest of tall eucalypts associated with Acacia simsii. On earlier occasions I had often walked over the flat rosettes of light green leaves and not recognised them. Further searching has revealed two other localities by the roadside in the dry open forest between Mt. Spec and Hidden Valley. In that area, early summer bushfires notwithstanding, leaf rosettes and the tiny 'trees' of delicate pink flowers were there on Easter Monday 1983 (April 4th),
The Townsville district specimens seem to belong all to the same species. The points of the spoon-shaped (spathulate) leaves are often prolonged into a thin hair. The flower spikes seen were up to 15cm tall. The flowers were pale pink with linear markings of deeper purple. The tiny seeds are held in small papery capsules. Viewed with a magnifying glass, the seeds appear rounded and of a medium brown colour.
It remains to identify the local species and to learn more of the plants' distribution. Trigger plants are probably more widely distributed in the Townsville district than this report indicates.
In the meantime, let me invite you - especially in summer and autumn - to come tripping triggers.
From the May 1983 issue of "The Native Gardener", the newsletter of the Townsville Branch of SGAP.
 [Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]
[Front Page] [Features] [Departments] [SGAP Home Page] [Subscribe]
Australian Plants online - March 1997
The Society for Growing Australian Plants
|