|
[Front Page] [Features] [Departments] [SGAP Home
Page] [Subscribe]

Propagation of the Quandong
Ben Lethbridge
This article describes simple, quick and efficient methods that I often use to grow my quandongs. They can be applied by most home gardeners.
Growing from Seed
Seed selection
Use only clean, fresh seed collected from a situation where cross-pollination is likely to have occurred. Seed of unknown age may not be viable and seed collected off the ground is usually badly contaminated with fungal spores. Seed derived from self pollination (ie isolated trees) usually produces plants of inferior quality.
Preparation of germination medium
I often had problems obtaining vermiculite of the right consistency to promote quandong germination. Either it was too wet or too dry or was contaminated with fungal spores, which was often associated with dry sterilization of the vermiculite. Wet sterilization and hot handling of the vermiculite alleviates these problems.
Soak the vermiculite in rain water, then drain it on a sieve until there is no free-draining liquid. Then place it on an oven tray, cover with aluminium foil and cook at 150 degrees C for 1 hour. While still hot (caution, steam!) transfer it to Zip-lock sandwich bags, seal and allow to cool overnight. The bags may be stored until required.
Seed Preparation
Either whole seed or kernels may be used. Germination of kernels is usually complete after two months but that of hole seed may take a year or more.
Kernel extraction
Some knowledge of the anatomy of the quandong seed (diagram) is needed for successful extraction of the kernel. Most of the diagram is self evident, but one important feature does require some explanation. The micropyle is a very fine tube connecting the inside of the shell to the outside. It is the usual means by which the seed absorbs moisture.
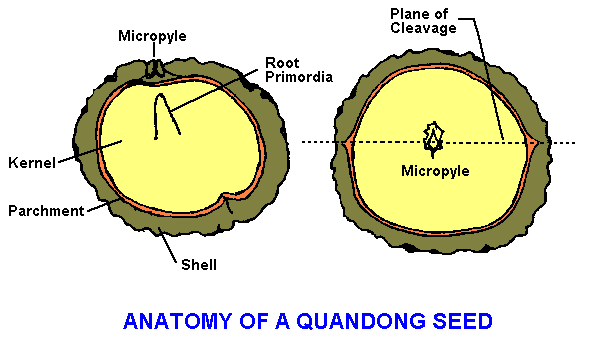
The micropyle is usually evident on the outside of the seed by a flat area with or without a symmetrical bump. It is obvious on examining a cross section of the remnant shell after whole seed germination. The micropyle is the weakest point of the shell and is the first point of the shell to break during germination. The micropyle also intersects a depression in the shell which bisects the entire seed. This natural weak point in the shell, which usually breaks during the germination process, can be utilised to efficiently extract whole kernels from whole seeds. Once you have identified the micropyle, place the seed in a vice with the micropyle directly against the vice jaws and very carefully place pressure on the seed. The seed should, more often than not, crack cleanly and the kernel can be removed. It is usually covered with a parchment layer that can be picked off with a thumb nail.
Seed sterilization, germination and growth
Place the seeds or kernels in a pot with holes in the bottom for drainage). Fill a larger container with 10 percent bleach (1 part household bleach : 9 parts water). Place the pot in it and agitate until the contents are completely wet. Soak for 30 to 45 minutes. Remove the pot and rinse the seeds or kernels thoroughly with cool, boiled rainwater. Place the seed directly in the vermiculite (about 10 per Zip-lock bag) and incubate in the dark at 15 to 23 degrees C (18 to 20 degrees is optimal). A.dark cupboard indoors is suitable. Addition of some fungicide to the whole seed preparation is suggested. Examine the bags after one week and remove any contaminated seed. Continue to examine twice weekly and remove germinated seed when the root radical is one cm long (approx 3 weeks for kernels, 2 months for seed). Either plant the germinated seed directly into the ground or pot on into as a large a pot as practicable.
Pot depth and size are important as the tap root of the quandong is very vigorous. In unrestricted growth situations over winter the shoot may only be a few centimetres long but the tap root can exceed 30 cm long. It is important to try to maintain the root system as close to natural as is possible. The smaller the pot, the more care and attention will be required to prevent loss of the plant while trying to establish it in the field.
Potting mix
Choose any low-phosphate free-draining potting mix. A potting mix made of peat moss and alkaline sand (1:4) supplemented with a low-phosphate slow-release fertilizer is suitable.
Host plants
Quandongs are semi-parasitic plants. This means that a host plant will be beneficial, but not essential, Any plant, even an already established one, will do. It is best to choose a plant that is not too vigorous as competition for water and/or nutrients can be a problem. It is important to get a good balance. I suggest a native perennial grass, legume, herb, shrub or prostrate species as suitable. It is perhaps advantageous to add the host to the pot before planting out as this may not only improve the parasitism but may also hold the root mass intact so that the quandong can be transplanted without root disturbance.
Establishment
People often describe quandongs as being adapted to growth and survival in arid to semi arid conditions. Direct-seeded quandongs may not require any special treatment. However, quandongs are not adapted to pot culture. When they are grown in pots, their root structure is drastically modified. These plants must be carefully nurtured after transplanting if they are to survive and thrive. The young quandong is quite succulent and preventing desiccation is important. Partial shade, supplementary watering, mulching and protection from strong hot and cold winds should be maintained until the plant has developed an adequate root system. It is suggested that quandongs be planted as early as possible into their permanent site. The best period is April to September (mid autumn to early spring). The soil should be well drained. Its pH is of lesser importance. I have found mulching and tree guards prepared from shadecloth to be beneficial to the establishment of pot-grown quandongs.
On the Graft
Why graft?
Grafting is a means of clonally propagating the above ground parts of dicotyledenous plants when the species does not breed true from seed, or cutting material doesn't easily produce roots. Grafting may be used to grow scion (above the graft union) onto a stock (below the graft) of a closely - related species which is more suited to the prevailing soil and climate.
Grafting and secondary growth
The growth of plants can be classified into two forms: Primary and Secondary. Primary growth is the growth that determines the height or length a plant attains . This growth is due to cell division of a small zone of the shoot. This zone is known as the apical meristem. Secondary growth is that growth which determines the girth or diameter of the plant stem. The lateral meristem or vascular cambium which is responsible for this growth occurs as a narrow band which runs along the length of the mature stem between (and giving rise to) the phloem and xylem elements. This narrow band, which is found between the bark and wood, is the "plumbing" of the plant.
The activity of the cambium from past growing-seasons gives the characteristic heartwood growth rings which are used in dendrochronology.
Plants that are produced by grafting (and cuttings for that matter) rely on active secondary growth; this is likely to be found in mature non-resilient (i.e. snappy not bendy) wood that is most often found in the previous season's growth.
The purpose of grafting is to join the cambial zone of the scion to that of the stock. When the cambial zones are brought into close contact, the resulting callus tissue that forms from metabolically active cambium will differentiate into connecting phloem and xylem tissue, thus re-establishing the severed "plumbing" and resulting in continued growth of the scion.
Grafting quandongs
Quandong cuttings callus readily but they do not produce roots. Quandong seedlings are quite heterogeneous (variable), so you can't be sure of getting a good plant from seed. However, I have successfully grafted suitable scion material onto seedling root stocks.
There are many different types of grafting techniques (see Elliot and Jones). The cleft graft is one of the most commonly-used and simplest types of grafts to perform. It is employed here for the grafting of quandongs.
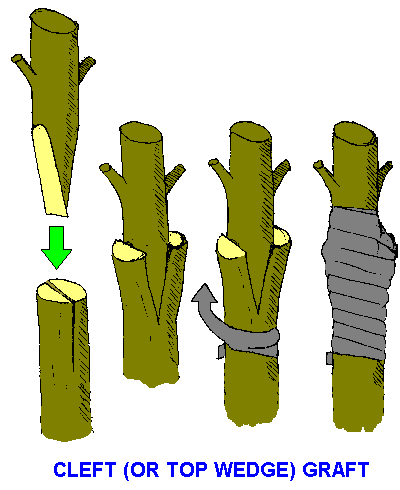 
Match the scion and stock diameters precisely; this maximises the chance of matching the cambiums.
The defoliated scion from a healthy plant should contain at least one completely dormant node on second-year wood which has had all soft, active growth removed. (Active soft growth will abscise, taking with it nutrients whose loss can be detrimental to the healing process and the success of the graft.)
The stock should be an actively-growing seedling (do the grafting during the warmer months) which is a few months old.
Cut the scion and fashion its base into a thin, narrow wedge. A large contact surface area will increase the rate of healing.
Cut the stock at right angles to the stem in mature wood or hypocotyl, preferably close to a node. Make a single vertical cut down the middle of the stem. The cut should be the same length as the wedge of the scion. Make sure that all cuts are straight and precise; use a very sharp knife or scalpel. Do not touch the cut surfaces, or allow them to dry out.
Force the wedge into the slit which was made in the stock; no gaps should be apparent.
Prepare a 0.5 cm-width piece of Parafilm or use shredded grafting tape.
Wrap the graft firmly with Parafilm, tying from just below the graft and working up. Care should be taken not to force the scion from the stock when traversing the join. Ensure that all points are covered with film. Air and water must be excluded from the graft-point if a successful union is to occur.
Cover the scion and graft with a small plastic bag (of the self-sealing variety) to minimize desiccation oft he scion. Place the plant in a stress-free environment such as a shaded (50-90%) hot house with temperatures that are suitable for vigorous growth of the quandong.
Examine regularly. The dormant nodes should burst in about 3 to 4 weeks. Residual petioles on the scion will abscise. Remove any buds that develop below the graft point.
Harden off the plant by gradually removing the plastic bag for increasing lengths of time or by cutting small holes in the plastic.
Remove the grafting tape at a later date.
References.
Raising from seed
- Australian Quandong Industry Newsletter, 1994. February, March and June.
- Bonney N, 1994. What Seed is that? Santalum acuminatum p. 271.
- CSIRO Division of Horticulture, 1991 The quandong.
- Grant WJR and Buttrose MS, 1978 SantaIum fruit, Australian Plants 9: 316-318.
- Lethbridge B, 1995, Current advances in quandong germination. SGAP Journal (South Australia)12: 370-371.
- Possingham J, 1986 Selection for a better quandong. Australian Horticulture Feb.: 55-59.
- Sedgley M, 1984 Quandong propagation Australian Horticulture Oct.:52-59.
- Your Garden,1994 Grow your own bush tucker. May: 40.
Grafting
- Beal, 1984 Australian Quandong Industry Association Conference 1994
- Botanic Gardens of Adelaide pamphlet, l992 Budding and grafting
- Elliot R and Jones D, 1980 Encyclopaedia of Australian Plants Suitable For Cultivation, Volume-l Chapter 20, "Grafting and Budding"
- Sedgley M, 1984 Australia's first native fruit, Australian Horticulture Oct.52-59
- Smith P, 1993 Australian Quandong Industry Association Conference 1993
- Taverna P, 1995 Australian Quandong Industry Association Conference 1995
Anyone interested in knowing more about quandongs should consider joining the Australian Quandong Industry Association by contacting the Secretary, AQIA, PO Box 236, Upper Sturt, South Australia 5156.
Ben Lethbridge has a PhD in plant sciences. He has a strong interest in the growing of quandongs and the species of the Adelaide area. This article is a composite of two articles which were published in the August 1995 and February 1996 issues of the Journal of the South Australian Region of SGAP.
 [Front Page] [Features] [Departments] [SGAP Home
Page] [Subscribe]
[Front Page] [Features] [Departments] [SGAP Home
Page] [Subscribe]
Australian Plants online - September 1997
The Society for Growing Australian Plants
|