
Native Plants and Phosphate
Jim Barrow
(From a talk given at ASGAP 2005 in Perth)
All life on Earth (and probably all life in the universe) needs phosphate (P). To say "Native plants don't need P" is nonsense. Many are very good at managing with little, and some are good at getting it. Indeed some are so unused to have much P in their environment that they do not have a mechanism for keeping out excess and so suffer form P toxicity.
However, small seeded plants that are capable of fast growth, usually respond strongly to P. Here (right) the response by Karri, which has a tiny seed, is similar to subterranean clover. On the other hand, large-seed plants and especially those with slow growth, such as some Proteaceae, may be capable of growth solely on seed reserves for many months.
Because P often determines rate of growth, controlling the supply is an important management tool for all ecosystem managers, whether the ecosystem is a small garden or a whole forest. Probably as many native plants have performed badly in gardens because of a shortage of P as have performed badly because of too much. Ergo, better learn something about it!
You need to know just one chemical fact about P: it loves oxygen. So keen is it, that it will burst into fire in air. So keen, that it grabs more than it is entitled to and surrounds itself with four oxygen atoms. The result is the phosphate ion: "PO4". Because it contains more oxygen than the valency of P can nicely balance, phosphate is negatively charged - actually there are three phosphate ions with different charge but the important thing from our point of view is that there is negative charge.
| You need to know just one thing about phosphate: it loves iron-oxide. That is why we use it to stabilise rusty surfaces prior to painting. |  |
| P is also very keen on aluminium oxides. As soon as phosphate ions are added to soil, most of them are grabbed by iron or aluminium oxide surfaces. |  |
| It is just about impossible to know how much of the P is stuck on iron oxides and how much on aluminium oxides. The two oxides have similar properties and, for our purposes it doesn't matter which is the more important. |  |
Because of this strong affinity, in most soils there is little P left in solution. So a soil might look a little like the diagrams below - with the yellow dots representing P ions and red monsters representing oxides.
Just how much depends on how much iron/aluminium oxide surface is present and on its properties. The more oxide, the more P you need to apply to have sufficient left in solution.
The amount of oxide varies widely - from very little in Perth grey sands to a great deal in soils derived from basic rocks (for example: basalt, basic gneiss) especially in areas of high rainfall. Thus the amount of sorption also varies widely. The graph below shows the effect of rainfall and of parent rock for soils from the Dorrigo to Ebor region of NSW.
The amount of iron oxides and therefore the amount of sorption is important for three reasons.
One is that the amount of P you need to get good growth is much higher on soils with a lot of iron/aluminium oxides - soils with high sorption. And a lot lower on soils with little iron/aluminium oxides.
You can see how big the difference in the amount of P needed can be on two different soils. When the soil with low sorption has reached maximum yield, the soil with high sorption has scarcely responded. Failure to appreciate such differences have caused many an agriculture scheme to fail. | 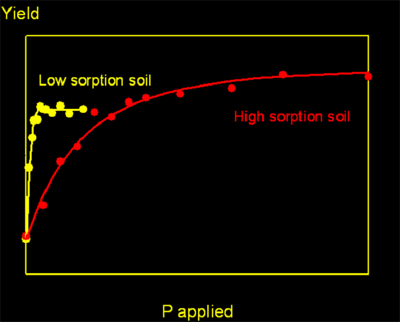 |
| Two is that the rate of supply is much slower on soils with high sorption - P is "rationed" to plants. This is shown right for sulfate, which to a certain extent follows the same rules as P. For a soil with little sorption (right), the plants quickly got all there was - and then ran out. For a soil of high sorption (left) the rate of supply was lower and the uptake curves are a different shape because the nutrient was "rationed" by the soil. | 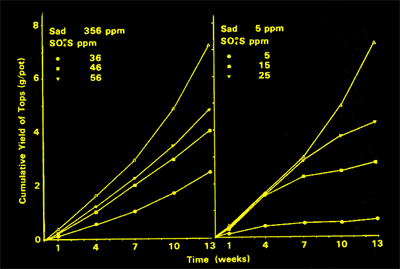 |
| And three is that whether P leaches into water bodies depends strongly on the soils sorption ability. In soils with high sorption, enormous amounts of excess P have to be applied before movement to water bodies is important. Soils are a bit like tanks. Those with little sorption fill quickly, but empty quickly too. | 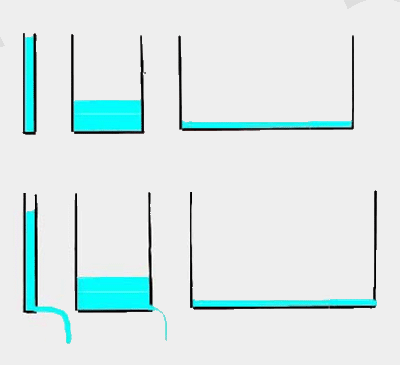 |
For a long time, many scientists thought that that was all there was to it. There was a small amount of P in the soil solution and a lot on the surface of the soil particles. But why does P fertilizer become less effective with time, and why do farmers re-apply P fertilizers? It is because there is a very slow continuing reaction between soil and P. P is so keen on iron/aluminium oxides that it likes to burrow in - the P slowly diffuses into the oxides.
This is a very slow process. After a year of reaction, the effectiveness of P fertilizer often decreases to about a half that of fresh fertilizer - and it gets even slower. That makes it difficult to study; science is seldom funded on such long terms. Fortunately we can speed the process by using higher temperature; by using say 60o we can cram a year's reaction into a day or so. The "buried" ions are not completely unavailable as they can slowly move back. But the further they have gone, the slower the return.
Consequently the effectiveness of P fertilizer decreases with time.
The graph below shows that the higher the temperature, the more P is needed to give the same yield. That is, the P has become less effective.
Perhaps the best way to deal with too much P is just to wait!
The phosphate ions that burrow into the particles take some of their negative charge with them and thus the particles become more negatively charged. Consequently they are less able to react with more P. It is this process that slowly leads to the soil becoming "saturated" with P and can, in soils with low to moderate sorption, cause leaching loss of P to water bodies.
That is the end of the story about soil. Now consider: how do plants take up P.
But that is not all either. We realised that some plants seem to be too efficient.
We now know that some plants secrete organic acids - such as citric acid.
This dissolves some of the iron oxides (and aluminium oxides) and releases some P.
If you are going to dissolve some P, it is a good idea to “wrap up” the affected bit of soil so the product of your industry doesn't get away. You develop “cluster” roots. These are sometimes referred to as proteoid roots but they are not confined to Proteaceae and indeed some of the early studies were with a species of Lupin.
This strategy is widely adopted by plants living on low-P soils and that means most plants in WA. But think about the long-term effects. Each growing season a flood of citric acid leaves the cluster roots and dissolves some of the nearby oxides.
The smaller particles are dissolved most quickly. Later in the season, soil microbes attack and oxidise the citrate so the oxides precipitate again, mostly around existing oxide particles. Hence the long-term effect of annual dissolution of oxides is, paradoxically, growth of ever larger lumps of oxide in our soils. It has been argued that this is why we have so much gravel in our soils and also the massive bands of oxides you see in parts of the landscape.
We had previously thought that our Proteaceae grew on our gravelly soils because they could. Now we are wondering if perhaps the soils are gravelly because Proteaceae grow there.
| |
 |
| |
Iron oxide layers near Wongan Hills, Western Australia |
Australian Plants online - 2006
Association of Societies for Growing Australian Plants
|