Chamelaucium Alliance – Background
Introduction
Chamelaucium is a well known genus in the myrtle family (Myrtaceae) through the very commonly cultivated species Chamelaucium uncinatum, the Geraldton wax. Within the myrtle family there are a number of genera which are regarded as having a close relationship with Chamelaucium, the genera in this related group being known as the Chamelaucium Alliance of the subfamily Leptospermoideae. All members of the subfamily are characterised by having a dry fruit but the factors that distinguish the Chamelaucium alliance from other members of the subfamily are not easily explained in simple terms.
Initially, the Chamelaucium Alliance was regarded as including those genera that had a a one-celled ovary (rarely two-celled) containing a single ovule (rarely two) in each cell, as shown in Figure 1 (the ovules are the unfertilized seed). A later classification extended the alliance to include several genera related to Baeckea. These genera have 2 or 3-celled ovaries which may contain two or more ovules, as shown in Figure 2.
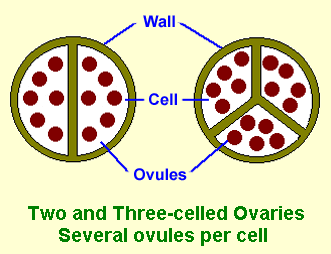 Figure 1 |
 Figure 2 |
| Adapted from Shrubby Myrtles by Rodger Elliot |
In the traditional characterisation of the myrtle family, there are about seven groupings or alliances within the subfamily Leptospermoideae. These include the Eucalyptus alliance and the Leptospermum alliance. The former includes Angophora, Corymbia and Eucalyptus while the latter includes a number of species and genera that are well known and in widespread culivation (eg Callistemon, Leptospermum, Melaleuca).
In recent years the traditional relationships within the myrtle family have been challenged and the somewhat neat arrangement of subfamilies and alliances within the family is now regarded as difficult to justify (see ‘Myrtaceae revisited: a reassessment of infrafamilial groups‘ in the tab titled Further Information for a technical discussion of the situation). Despite this, the plants within the traditional view of the Chamelaucium Alliance do form a consistent and recognisable grouping. For this reason, and for simplicity and convenience, the term “Chamelaucium Alliance” will be used here – the botany will sort itself out in due course…..
Taxonomy changes in Baeckea
The genus Baeckea is one of the better known members of the Chamelaucium Alliance. However, in recent years there has been been considerable reclassification of species in that genus with the result that many species that were formerly classified as Baeckea are now classified under several new or re-instated genera including Babingtonia, Euryomyrtus, Harmogia, Kardomia, Ochrosperma, Sannantha and Triplarina. This means that Baeckea itself has been much reduced in size.
One very well known species that has been affected by these changes is Baeckea virgata, a small to large shrub which has been widely cultivated. In several papers published in the 1990s, Tony Bean of the Queensland herbarium revised the genus Baeckea and one result was the change of Baeckea virgata to Babingtonia virgata. However, a second consequence of Bean’s work was that he regarded Babingtonia virgata as being restricted to New Caledonia – i.e. under Bean’s revision Babingtonia (Baeckea) virgata is not an Australian native plant. This meant that all Australian plants previously allocated to Baeckea virgata required new names and Bean described 8 new Babingtonia species to cater for this Baeckea virgata complex. The article “When is a Baeckea not a Baeckea” in the December 2001 issue of Australian Plants online explains the situation in more detail.
 |
| Sannantha similis, previously classified as Babingtonia similis and before that as a form of Baeckea virgata. Photo: Brian Walters |
However….
While Bean’s work and classifications were adopted by most authorities, research into Baeckea and closely related genera, particularly Babingtonia, continued. In a paper Re-evaluation of the genus Babingtonia (Myrtaceae) in eastern Australia and New Caledonia (Wilson P G, Heslewood, M M and Quinn, C J; Australian Systematic Botany 20, 302-318, September 2007), the authors concluded that the genus Babingtonia should be considered restricted to Western Australia where it encompasses a relatively small group of species. They described two new genera, Sannantha and Kardomia, and reinstated a third genus, Harmogia, to cater for the other species of Babingtonia previously included under Bean’s classification.
This new work has been adopted in the Australian Plant Census.
(Subsequent to the above, the New Caledonian species has been re-classified to Sannantha virgata.)
Members of the Chamelaucium Alliance
As already noted, the best known species in the Chamelaucium Alliance is the Geraldton Wax (Chamelaucium uncinatum). This is extremely popular in cultivation for its long lasting, waxy flowers which are excellent for cutting. Numerous cultivars of this species have been developed both in Australia and overseas. Other members of the alliance which are commonly (or occasionally) seen in cultivation include Baeckea, Sannantha, Thryptomene, Verticordia, Homoranthus and Darwinia.
The following tables list the genera in the alliance as well as the number of species in each genus and the distribution of each genus by state. Note that there is still active research being undertaken within this group of plants and the number of species within each genus can only be approximate.
The list is based mainly on information from the Australian Plant Census, supplemented by information from the Western Australian Florabase data for Enekbatus and Tetrapora. It also acknowledges the reclassification of Babingtonia, previously mentioned.
Genera in the Chamelaucium Alliance
|
Genus
|
No.of
Species* |
Distribution
|
|---|---|---|
|
Actinodium
|
|
WA
|
|
Aluta
|
|
WA
|
|
Calytrix
|
|
All states
|
|
Chamelaucium
|
|
WA
|
|
Corynanthera
|
|
WA
|
|
Darwinia
|
|
All mainland states
|
|
Homalocalyx
|
|
Qld, NT, WA
|
|
Homoranthus
|
|
Qld, NSW, SA
|
|
Malleostemon
|
|
WA
|
|
Micromyrtus
|
|
All mainland states
|
|
Pileanthus
|
|
WA
|
|
Thryptomene
|
|
All states
|
|
Verticordia
|
|
WA and NT
|
| * Approximate number only; some genera contain numerous unnamed species and other genera are in need of, or are undergoing, botanical revision. | ||
|
Genus
|
No.of
Species* |
Distribution
|
|---|---|---|
|
Anticoryne
|
|
WA
|
|
Astartea
|
|
WA
|
|
Astus
|
|
WA
|
|
Babingtonia
|
|
WA
|
|
Baeckea
|
|
All states except SA
|
|
Balaustion
|
|
WA
|
|
Cyathostemon
|
|
WA
|
|
Enekbatus
|
|
WA
|
|
Euryomyrtus
|
|
All states except Qld
|
|
Harmogia
|
|
Qld, NSW
|
|
Hypocalymma
|
|
WA
|
|
Kardomia
|
|
Qld, NSW
|
|
Ochrosperma
|
|
NT, Qld, NSW
|
|
Rinzia
|
|
WA
|
|
Sannantha
|
|
Qld, NSW, Vic, New Caledonia
|
|
Scholtzia
|
|
WA
|
|
Tetrapora
|
|
WA
|
|
Triplarina
|
|
Qld, NSW
|
| * Approximate number only; some genera contain numerous unnamed species and other genera are in need of, or are undergoing, botanical revision. | ||
Characteristics of Chamelaucium and its Relatives
Plants in the Chamelaucium Alliance are usually small to medium sized shrubs often with small (sometimes minute) leaves. Like other members of the myrtle family, the leaves contain aromatic oils which can be smelled by crushing the leaves between the fingers. In some cases the aroma can be quite ‘sweet’, as in Chamelaucium uncinatum, or citrus-like, as in Darwinia citriodora. The flowers are quite variable; many have five-petalled flowers superficially similar to those of the ‘tea tree’ (Leptospermum sp.) which can be quite large, as in the case of Geraldton wax or tiny, as in some Micromyrtus and Thryptomene species. In other cases, the individual flowers may be quite small and inconspicuous but are clustered together and surrounded by large, colourful petal-like bracts (eg. some Darwinia species). Still others have conspicuous sepals with conspicuous hairs on their margins (Verticordia species).
Insects appear to be the main pollination vectors for the Chamelaucium Alliance. However, birds are also attracted to some species, particularly in the genus Darwinia. This is especially noticeable with the bell-shaped inflorescence of some members of that genus (eg. D. leiostyla, D. macrostegia, D. meedoldii)
The majority of species in this group of plants are found in heath, woodland or open forest of mainly temperate areas. They are absent in rainforest and arid areas although some species do occur in the tropics.
The general features of each genus are described in the following table. The table is not intended to be a botanical ‘key’ as differences between the genera may not always be apparent to the casual observer, requiring botanical knowledge and a high magnification viewer.
Table 2 – Some Characteristics of Chamelaucium and its Relatives
|
Genus
|
Characteristics
|
|---|---|
|
Actinodium
|
Small shrubs; inflorescences terminal (occurring at the ends of the branches) and ‘daisy-like’ in appearance.
|
|
Aluta
|
Small shrubs; genus split off from Thryptomene in 2000 and very similar in appearance to that genus. Five-petalled flowers occurring in the leaf axils (axilliary) with petals persistent.
|
|
Anticoryne
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Astartea
|
Small shrubs with five-petalled flowers in the leaf axils; similar to Baeckea but differing in the arrangement of the stamens.
|
|
Astus
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Babingtonia
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Baeckea
|
Small to large shrubs. Small, five-petalled flowers in the leaf axils. Baeckea also occurs outside of Australia.
|
|
Balaustion
|
Small genus of 2 species. Small shrubs with flowers occurring singly in the leaf axils. Base of the flower is distinctly inflated.
|
|
Calytrix
|
Small to medium shrubs. Most Calytrix have long hairs or awns on the tips of the calyx and the flowers nearly always occur in terminal clusters at the ends of the branches. Calytrix includes species previously classified as Lhotzkya and Calythropsis and differs from Homalocalyx in having persistent (not deciduous) sepals.
|
|
Chamelaucium
|
Small to large shrubs; waxy, five-petalled flowers occurring in the leaf axils and often larger than other members of the alliance. Some species are commercially exploited for cut flowers.
|
|
Corynanthera
|
Monotypic genus. Corynanthera flava is a small shrub similar to Micromyrtus but having inflorescences in terminal, elongated spikes.
|
|
Cyathostemon
|
Small to medium shrubs previously included in Astartea and similar to that genus.
|
|
Darwinia
|
Small shrubs; small flowers with short petals and an elongated calyx tube terminating in 5 small petal-like lobes. Flowers in pairs or up to 30 in a compound head, sometimes surrounded by leafy bracts.
|
|
Enekbatus
|
Small to medium shrubs previously included in Baeckea and similar to that genus. This genus is yet to be formerly published but the classification has been adopted in Western Australia.
|
|
Euryomyrtus
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Harmogia
|
Small to medium shrubs previously included in Babingtonia and similar to that genus.
|
|
Homalocalyx
|
Small shrubs similar to Calytrix but having petal-like, deciduous sepals – includes all species previously included in Wehlia.
|
|
Homoranthus
|
Small shrubs similar to Darwinia but calyx lobes feature single or multiple hair-like awns.
|
|
Hypocalymma
|
Small to medium shrubs. Closely related to Astartea and Cyathostemon.
|
|
Kardomia
|
Small to medium shrubs previously included in Babingtonia and similar to that genus.
|
|
Malleostemon
|
Small shrubs. Genus split off from Micromyrtus and Thryptomene with characteristics similar to both of those genera.
|
|
Micromyrtus
|
Small shrubs similar to Thryptomene. Five-petalled flowers occurring in the leaf axils (axilliary) with petals usually deciduous.
|
|
Ochrosperma
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Pileanthus
|
Small to medium shrubs similar to Chamelaucium but having 20 stamens rather than 10. Flowers occur in corymbs at the tips of the branches.
|
|
Rinzia
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Sannantha
|
Small to medium shrubs previously included in Babingtonia and similar to that genus. Sannantha also occurs outside of Australia.
|
|
Scholtzia
|
Small to medium shrubs with five-petalled flowers similar to Baeckea.
|
|
Thryptomene
|
Small shrubs with five-petalled flowers occurring in the leaf axils (axilliary) and having persistent petals.
|
|
Tetrapora
|
Small to medium shrubs. A reinstated genus, similar to Baeckea.
|
|
Triplarina
|
Small to medium shrubs previously included in Baeckea and similar to that genus.
|
|
Verticordia
|
Small shrubs similar to Darwinia and Homoranthus but calyx lobes have feather-like hairs, giving rise to the common name “feather flowers”.
|
Chamelaucium Alliance – Propagation
Introduction
Propagation of plants in the Chamelaucium Alliance from seed is often unreliable and propagation by cuttings is the preferred method. This also enables plants with desirable characteristics of form or flower colour to be perpetuated. Some species, particularly Darwinia and some Verticordia, have been successfully propagated by grafting.
Seed
While germination of seed of this group of plants is not impossible, seed of many species is often difficult to obtain because, in many cases, the seed is released from the mature capsules when ripe, making it difficult to collect. Germination data on many species is also limited or not available. Seed of some species of the following genera can usually be obtained from commercial suppliers, and some others may be available from time to time.
- Baeckea, Sannantha, Babingtonia and (presumably) related plants such as Kardomia – usually germinate reliably without pretreatment.
- Calyrtix – the best results seem to occur if the seed is pre-germinated prior to planting into seed raising mixes. Pre-germination involves placing the seed in an enclosed container together with moist paper or vermiculite until the root is seen emerging from the seed. This may take 2-3 weeks or more (refer to ANPSA’s Plant Propagation Pages for more information).
- Chamelaucium – germination unreliable.
- Hypocalymma – usually germinates reliably without pretreatment.
- Thryptomene – usually germinates reliably without pretreatment.
- Verticordia – germination unreliable.
Another method that has been successful for at least some species is the use of smoke or “smoked water” as a pretreatment. This has been successful in the germination of species of Astartea, Baeckea, Calytrix, Hypocalymma and Verticordia and may have practical application for the home propagator. Further information on this procedure is available in the article Smoke Stimulates the Germination of Many Western Australian Plants (see the Further Information tab) and from the Regen 2000 web site.
Cuttings
Many plants in the Chamelaucium alliance are readily propagated by cuttings using hardened, current-season’s growth. Cuttings about 75-100 mm in length, taken in January in southern Australia would normally be suitable with the leaves carefully removed from the lower two-thirds. “Wounding” the lower stem by removing a sliver of bark and treating with a “root promoting” hormone both seem to improve the success rate.
Grafting
Because some of the most spectacular members of the Chamelaucium alliance are native to the Mediterranean climate of south-west Western Australia, attempts at cultivation in humid areas of the east coast are not always successful. Particularly difficult are the spectacular ‘feather flowers’ (Verticordia species) and the darwinias in the ‘mountain bell’ group. To try to expand the range where those plants can be successfully cultivated, some work has been carried out on the grafting these species onto hardy rootstocks.
This work has been successful to a limited degree. Darwinia citriodora seems to be the root stock of choice for grafting of Darwinia and Verticordia species but only in districts where D.citriodora is reliable (despite the fact that D.citriodora is itself a species from the south-west, it is hardy in temperate areas of the east coast). Grafted plants of species such as D.meeboldii, D.macrostegia and D.oxylepis are now commonly available at specialist native plant nurseries.
The hardiness of Chamelaucium uncinatum varies not only according to climate but also according to clone, whith some forms being noticabaly more hardy than others. For example, the common pink flowered form is much hardier than the deeper coloured forms in temperate and sub-tropical areas. Grafting of less hardy forms onto a rootstock of the hardy, pink flowered form should enable those less hardy forms to be cultivated more widely. Other rootstocks for Chamelaucium that have been tried with some degree of success include Darwinia citriodora, Kunzea flavescens, Kunzea ambigua and Leptospermum petersoni.
There is still scope for enthusiastic amateurs to carry out rootstock trials for plants in the Chamelaucium alliance. There are two factors to be considered when selecting suitable rootstocks:
- Hardiness of the stock – the selected stock needs to be reliable in the area where the grafted plant is to be grown.
- Compatibility between stock and scion – the closer the relationship between the stock and scion, the better the chance of success. Stock and scion of the same species is ideal, stock and scion in the same genus is often successful and stock and scion in closely related genera may also be suitable if other options are not viable.
General Propagation
Further details on general plant propagation can be found at the Society’s Plant Propagation Pages.
Chamelaucium Alliance – Cultivation
Introduction
With so many species within the Chamelaucium alliance, and such a wide natural distribution covering a range of different climatic zones, it is probably not surprising that problems may be experienced when trying to grow a species in a different climate to that of its natural habitat. As a general guide, species native to south western Australia where dry summers and wet winters are experienced may prove unreliable in tropical and sub tropical areas and in temperate zones which experience wet, humid summers.
Unfortunately, as the majority of species in the Chamelaucium alliance do occur in the south west, most are a challenge under cultivation east of the Great Dividing Range, unless grafted on to a hardy root stock. Despite this, a number of species have proven to be adaptable in seemingly unsuitable climates. For example, the Geraldton wax, Chamelaucium uncinatum, can be successfully grown in coastal New South Wales and south-east Queensland (although the normal pink flowered form is much easier to maintain than the purple and maroon forms). Darwinia citriodora is also reliable in the south east, which is why it is often used as a root stock for some of the less easily grown species.
As a general rule. plants in the Chamelaucium alliance require medium to light soils (eg. sandy loam) that are well drained but which retain some moisture. They are unlikely to survive for any length of time is continually wet or boggy conditions. Most require a sunny or lightly shaded position for best flowering.
Most species respond favourably to a light annual trim to promote bushy growth but severe pruning below green foliage should be avoided.
There are few pests that cause serious problems with this group of plants except that western species may be sensitive to root rot fungus (Phytophora sp.), which is one reason why they can be difficult to grow under humid summer conditions. Other than that, scale is the most likely pest that may occur. This can be controlled either by physical removal (on smaller plants) or treatment with pest oil on larger specimens.
Most of this group of plants occur in soils that are nutrient deficient and excessive fertilising can be detrimental. The use of a slow release fertiliser after flowering will usually be sufficient to maintain healthy growth.
Further information on the cultivation of Calytrix and Verticordia is available via the main Geraldton Wax and Relatives index.
The following table lists some of the species that have proved reliable in a range of climates but, as a general rule the range of species that can be grown successfully is greatest in southern Australia and decreases as the climate changes from temperate to subtropical to tropical, east of the Great Dividing Range. If you want to grow some of the most spectacular chamelauciums and verticordias, you may need to move to the south-west….
Reliable Members of the Chamelaucium Alliance
|
Genus
|
Species
|
|---|---|
|
Astartea
|
fasicularis, ‘Winter Pink’
|
|
Baeckea
|
diosmifolia, gunniana, linifolia
|
|
Calyrtix
|
acutifolia, angulata, fraseri, tetragona
|
|
Chamelaucium
|
floriferum, uncinatum
|
|
Darwinia
|
citriodora, fasicularis, procera, taxifolia; several Western Australian species are available as grafted plants (eg. leiostyla, macrostegia, meeboldii, oxylepis)
|
|
Euryomyrtus
|
ramosissima (often still listed under Baeckea)
|
|
Harmogia
|
densifolia (often still listed under Baeckea or Babingtonia)
|
|
Homoranthus
|
flavescens, porteri, virgatus
|
|
Hypocalymma
|
angustifolium, cordifolium ‘Golden Veil’, xanthopetalum
|
|
Micromyrtus
|
ciliata
|
|
Sannantha
|
tozerensis, similis ‘Howies Feathertip’ (all often still listed under Baeckea or Babingtonia)
|
|
Triplarina
|
imbricata (syn. Baeckea camphorata)
|
|
Thryptomene
|
denticulata, saxicola ‘Paynes hybrid’
|
|
Verticordia
|
chrysantha, densiflora, grandis, monadelpha, plumosa
|
| Note: The various forms of the popular garden plant formerly called Baeckea virgata are now regarded as belonging to the genus Sannantha. However, they are NOT classified under Sannantha virgata and there is still confusion as to their correct taxonomy. | |
Cultivation in Containers
Many of this group of plants make ideal subjects for containers because of their modest size. This is also a good way to grow some of the species that are difficult to maintain under garden conditions such as some of the colourful western Calytrix, Darwinia and Verticordia.
For container cultivation choose a quality, free-draining mix, preferably one designed for Australian native plants as these will not have excessive amounts of nutrients. Plastic or ceramic pots are both suitable although watering requirements will differ as non-glazed ceramic pots are porous and will dry out quicker than glazed or plastic pots. The pot should not be large enough to cater for about two season’s growth before re-potting is required – a pot of about double the volume of the previous pot is large enough. If the chosen pot is too large, controlling moisture content within the mix can be difficult leading to the development of over-wet areas and the risk of root rot.
The key to successful container cultivation is to not over-water. Before watering, check the soils a few centimetres below the surface – if the mix feels wet, then watering is not needed. Fertilising is probably more important for container-grown plants than for those in the garden as nutrients can be leached out of the potting mix. Even so, resist the temptation to over-fertilise – light applications of slow-release fertiliser during the active growing period is sufficient.
Growing Calytrix
Gwyn Clarke
This article is a 2001 update of Gwyn’s original article Growing Calytrix published in the Society’s journal Australian Plants, June 1990. Gwyn is a former leader of ANPSA’s Calytrix Study Group.
The Calytrix or ‘star flowers’ are rarely seen in gardens, except for Calytrix tetragona, and most people would not realize that there are over 70 species growing in the wild. They are quite spectacular when in flower and have colourful calyces to follow. The most commonly grown species, C.tetragona is very widespread and variable. It is found in woodlands and forests from southern Queensland to south Western Australia. The white form is most commonly seen but pink forms are also found, particularly in western Victoria. Other species may be available from time to time at specialist nurseries.
Why then have these plants not been seen more often in cultivation?
 |
 |
| Calytrix tetragona – white and pink forms Photos: Brian Walters |
Availability
Since many of the species come from Western Australia obtaining plant material has not been easy. Seed has generally proved to be of low viability. Perhaps the seed was old, had dormancy factors or germination inhibitors or sustained considerable insect damage. When seed has germinated it has been slow to grow on and has had poor root systems. Cutting material has given greater success as far as eastern States’ species are concerned. When material from Western Australia has been obtained it has been generally more difficult to strike, and certainly more difficult to maintain plants in the ground, however pot culture is a different story.
Experimenting with Western Australian Seed
I purchased seed from Nindethana Seed Company in Western Australia. The seed of Calytrix is indehiscent with calyx and awns present. The calyx and awns were removed, and the seed pushed into the soil point first. The top of the seed should be level with the soil. The seed was watered by lowering the seed punnet into the water until water level was at the top of the punnet, the seed punnets were then placed in a capillary bed.
Air movement around the seed punnets was important as seedling Calytrix are very prone to fungal attack. The seedlings were kept inside the house with underfloor heating operating. The temperature in the room varied between 15 and 23 degrees C. This was done because it was winter in Canberra and I had no other suitable facilities.
Germination time was very variable, C.angulata appearing after six weeks and C.aurea after 13 months. Plants should be potted on as soon as practicable.
I had success germinating the following species: C.angulata, C.glutinosa, C.leschenaultii, C.exstipulata, C.fraseri and C.depressa. C.aurea only produced one seedling which died before reaching a usable size.
The seedlings were potted on and there were the usual losses. Most of the living seedlings grew until they were about 2.5 cm high. Then they just sat there. Some damped off and I was afraid I was going to lose all of them, but a suggestion from a friend helped save some of them – see below. (I should mention that I haven’t really spent much time working on seed of eastern states species. This is because they are generally easier to get hold of and grow very well from cuttings. I have certainly had seedlings of C.tetragona popping up in my garden.)
Experimenting with Cuttings
Lyn Craven suggested I take cuttings from the above seedlings as this might help break their dormant state. This proved to be the case. The cuttings taken were 2 cm long, with about 4-5 pairs of leaves. They were prepared in the usual way, care being taken not to damage the delicate stem. They were placed around the edge of a black pot in a mix of 2/3 washed river sand and 1/3 peat. The pot was then placed in the hot bed, (by this time I didn’t need my underfloor heating anymore).
Within 10 days most of the cuttings had roots and were potted up. I used the hot bed because this material was quite soft. That was in January 1985. Most of those plants lived for 7-8 years in the garden; Calytrix angulata is still alive and flowers each year in a raised bed. The others were quite happy in pots for even longer and some were given to the new leaders of the Calytrix Study Group when I retired. All the plants eventually flowered. Plants included the following species, C.angulata, C.glutinosa, C.leschenaultii and C.depressa.
 |
 |
| Top: Calytrix leschenaultii Bottom: Calytrix drummondii Photos: Brian Walters & Geoff Clarke |
Since first writing this article in 1990 I have done a lot more experimenting with cuttings, and while the information for using soft young growth remains the same, cuttings can be struck from older material.
Cuttings of Calytrix may be taken from young growth before it becomes woody. Small cuttings, i.e., 4-5 cm, do very well, but they can be longer. I have used liquid hormone, 1000 ppm IBA/250 ppm NAA, but have also had good results without hormone. Cutting mix was 4 parts washed river sand, 4 parts perlite, 1 part peat. However, any well drained and aerated mix will do. Some will do well in the hot bed, but many prefer open shade with low humidity even though they take a little longer to form roots; e.g. C.longiflora from Queensland, and many forms of C.tetragona. Timing is also important, and I have found December/January an excellent time in Canberra, but cuttings may be taken as late as April.
Success may also be had with woody cuttings. They respond well to IBA4000 and open shade. It may be that different times are more appropriate in warmer climates. The important thing is to be flexible and experiment to find the best conditions where you live.
Calytrix in the Garden
Interesting species in this genus have often proved short lived in cultivation, and susceptible to grey mould. However, given the correct conditions, Calytrix can do well and live for a reasonable length of time. The eastern states species, (many forms of C.tetragona, and C.alpestris), are quite long lived and even some of the Western Australian species have lived 7-8 years. C.acutifolia and C.angulata from the west are 10 and 15 years old respectively. In more humid climates than Canberra the fungal diseases may pose more of a problem.
Calytrix like good drainage, in sun or semi-shade and enjoy living close to other species. They also like a spot with plenty of air movement as this helps prevent grey mould. I have mine in garden beds with gypsum and washed river sand added, or in raised beds made of washed river sand, peat, compost and local clay. Raised beds are particularly important for species from Western Australia. All the beds are mulched with sand or an open mix of stems twigs and leaves. This allows good aeration round the stem and prevents collar rot. Some mulches pack down hard around the stem and should be avoided.
Calytrix should also be pruned lightly and regularly to avoid woodiness. If a plant should become woody, heavy pruning can restore vigour. Plants doing well in the described environment include upright, procumbent and prostrate forms of C.tetragona, and C.alpestris, C.angulata, C.acutifolia, and C.depressa.
Pot Culture
Many species make excellent pot plants. All those I have in pots are doing well. They do not require repotting too often (about every two or three years) and respond well to regular watering with half strength Aquasol, or other liquid fertilizer. You do need to be careful with species that like sun and good air movement. I recently lost two C.longiflora from fungal attack because they were in a shady protected spot when they really needed full sun. My son’s plant is doing well in a very sunny courtyard.
Horticultural Potential
 |
| Colourful calyces of Calytrix tetragona Photo: Brian Walters |
Calytrix, with their dainty star flowers are really worth growing, and with a little care they can be propagated and maintained in the garden. They are outstanding in flower although the flowering period can be quite short, but the colourful calyces which follow are as delightful as the flowers themselves.
We are only at the beginning of the development of these plants for horticulture. There are still many things to be discovered, and many experiments to be attempted. Cross pollination between species could produce plants with larger flowers or extend flowering times. The robust qualities of Calytrix tetragona could perhaps be passed on to its more fragile relatives. Grafting, budding and tissue culture have yet to be attempted.
Anyone can be part of the development of and the experimentation with this genus. These little gems will reward you with their colourful flower displays, bright calyces and scented leaves. I have even found seedlings growing happily under the parent plants. My latest is a low growing form of C.tetragona with a large, soft, pink flower. Its parents – a prostrate pink form with tiny crowded flowers and a tall white form (3m high) with large white flowers. What greater reward could you ask?
Verticordia in the Garden
Max Hewett
The late Max Hewett was the leader of ANPSA’s Verticordia Study Group. Although this article was written in 1995, the information is still relevant for those wishing to grow Verticordia species. The article was first published in the Society’s journal Australian Plants, Vol.18 No.145, December 1995.
Introduction
Except for a few tropical species which occur across the top of Australia and which to date have resisted establishment in the southern states, Verticordia is endemic to the south-western winter-wet corner of the continent. As might be expected, their introduction to areas with opposing weather patterns is not without problems. However, until comparatively recent times, very few species have been successfully established even in their own climatic region.
 |
| Verticordia picta Photo: Brian Walters |
As with many other examples of Western Australia’s outstanding flora, once thought to be difficult because of climatic variation, but now becoming well known in the summer-wet regions of eastern Australia, the growing of Verticordia may now be contemplated by those prepared to give that little extra thought and effort.
It has become evident that many parameters are common to both summer and winter-wet areas. Many species are now being grown successfully throughout Australia. With others, we have had only partial success but this will serve as a basis for more research into better long-term establishment.
Grafting treatment has also proved very worthwhile for some species which have shown sensitivity to soil-borne pathogens. More general commercial application will increase the popularity of these beautiful wildflowers, as they could then be grown without provision of special conditions.
Many cultural matters influence plant performance of species from Western Australia when grown in the eastern states, such as fertilising, time of planting in various climatic zones, response to dry winter and early spring conditions, especially in summer-wet areas, plant aspect, etc. Early research has been directed predominantly towards identification of the basic major hazards.
Major hazards in growing Western Australian plants, especially Verticordia
These hazards are recognised as root rot, collar rot and mildew, moulds etc. Chemical treatments may be appropriate at times but research to date has concentrated predominantly on the achievement of control by physical considerations.
- Root rot of garden shrubs
Root rot is destruction by soil borne pathogens which attack root structures. It results in the plant’s inability to take up moisture and nutrients from the soil and has confronted gardeners from time immemorial. With specific attention to soil type and drainage, this hazard can be controlled reasonably well. It is a particular problem with some Verticordia species in summer-wet areas.The traditional gardening response has been to improve drainage conditions, and mounding of beds has certainly proved worthwhile. With some Verticordia, particularly in late summer when the soil is warm and moist, inadequate rate of drainage as well as the depth can be a significant factor. Compacting of soils over time, particularly with soils in the medium to heavy range, may be a problem. Initial planting procedures can be important and my recommendations are given in the table under ‘Cultivation of Verticordia‘, below. Some species such as Verticordia chrysostachys var. pallida have made very good early growth in finely textured, well mounded loam but slow drainage speed has proved inadequate in late summer. Verticordia picta, is reliable in light sand or sand and gravel to a depth of about 30 cm over heavy base, but has been difficult to establish otherwise.A reasonable guide to preferred soil type may be assessed from observation of the initial root development of species at the propagation stage. Where first roots emerge predominantly horizontally or at least shallow, it is probable that the species will do well in heavy soils. Varieties of Verticordia huegelii in this category have adapted well in quite heavy clay loams. Where the first root development tends to be predominantly vertical, lighter soils have generally proved more appropriate. Some Verticordia species may develop a lignotunerous root system and are less troubled by problems of root rot. Verticordia chrysanthella is one such species which has done well in both light and heavy soils. - Collar rot of garden plants
Collar rot is attack by soil-borne pathogens to the plant stems, at or near surface level. Destruction of the outer section of the stem (known as the cambium layer) results in the inability of the plant to transfer moisture or nutrients between the root system and the foliage. In summer-wet areas such as eastern Australia this hazard can be a major problem with many garden shrubs, especially many western plants such as Verticordia that have evolved in a summer dry climate.

Top: Verticordia pritzelii
Bottom: Verticordia grandiflora
Photos: Keith Townsend & Brian Walters - Factors affecting collar rot include mulching of the soil surface around the plant, watering methods and for Verticordia, in my opinion, potting mixes and plant dormancy. Of these, the surface condition of the soil is the more apparent difference between growing in summer-dry and summer-wet areas. In the west, the summer dry period, as well as extending well into autumn, is generally hotter, and the hazard of collar rot is reduced. This is the establishment period of young plants, and vegetative surface mulches created by leaf litter are an advantage in such areas.In summer-wet areas, vegetative mulches appear to be a source of collar rot attack. The adoption of more sterile surface conditions using gravel mulches or by permitting undisturbed natural weathering in the case of gravelly soils, has done much to alleviate this collar rot problem.Many Verticordia flower from late spring well into summer and before flowering they will respond favourably to summer rain or hand watering. Flowering is frequently followed by some degree of dormancy and the humid late-summer conditions which prevail in eastern areas coincides with this rest period. This can place collar rot susceptible species under additional stress.It has been observed with some species, in the higher range of susceptibility to collar rot attack, that failures have occurred due to the moisture-holding capacity of the potting mix used initially in the nursery stage. It has seemed that this has later contributed to collar rot problems in holding more moisture near the plant stems than is desirable during summer-wet conditions. My solution to this is given under the heading ‘Planting Procedure’ below.To maintain sterile surface conditions around planted specimens, it is sometimes necessary to clear the plant’s own leaf litter away from plant stems should excessive summer leaf drop occur. Verticordia staminosa ssp. cylindracea var. erecta is one such species which, although generally quite hardy and reliable, can be vulnerable to attack under wet late-summer conditions. Growth response to wet weather throughout the year is good with dense leaf production. It is one of the earlier species to flower in dry conditions in early summer and when flowering has finished, heavy leaf drop can lead to collar rot attack.Hand watering can increase the risk of attack with some species. Verticordia have shown very good tenacity under hot and dry summer conditions and mature specimens, particularly in eastern Australia, have generally proved quite capable of surviving without such attention. An exception can arise where very shallow rooted species have been grown in light sandy soil and the entire root system is in danger of drying out. Watering should be maintained during the establishment stage should dry conditions prevail. In the cooler part of the year hand watering may safely be done throughout the day. When species are in flower it is best to water below the foliage to avoid flower damage. During hot weather water in early mornings so that excessive moisture is not retained around plant stems for long periods. Do not water late or overnight, when most collar rot problems start. It is also important that watering be done on occasions when the weather conditions later in the day could be expected to dry the surface quickly such as from wind, hot temperatures or low humidity. Trickle watering has sometimes been used; in light sandy beds it can be difficult to obtain adequate moisture distribution through the soil and spraying may be more appropriate.Mound specimens slightly at planting time, and form slight depressions between plants. As well as helping to throw excess water away from the vulnerable stem areas and thereby effect faster surface drying after rain, the depressions can also be useful in training water deeper into the soil where it may do more good. Such treatment furthermore tends to reduce general surface scour in heavy rain.
- Mildews, moulds, etc.
Fungal attack on the stems or leaves is a hazard when cultivating some plants, resulting in plant debilitation and, at times, death. This hazard can be more difficult to control than root and collar rot and grafting on to hardier rootstock does not avoid the problem. Attack appears to be related more to seasonal conditions than to geographical division into summer or winter-wet regions. On some plants the effects are relatively minor and may only cause temporary damage even if no counter measures are taken.

Photo: Brian Walters
For Verticordia, fungal attacks occur more frequently in summer-wet areas and in wetter than usual summer conditions in other areas. In summer-wet areas some of the yellow-flowered species are particularly susceptible in autumn to mildew attack, although when late summer weather patterns are drier than usual the problem is much less evident. Verticordia chrysanthella is such a species and although debilitation may be quite appreciable in some years, with defoliation, recovery may generally be expected later during winter and spring. It appears to be caused, not so much by the wet, late summer conditions to which the plant may have responded with lush new leader growth, but to the effect on such growth a little later in autumn, from widely-contrasting drier weather, and while night temperatures are still moderate. One approach is to do nothing and await natural recovery. Healthy new growth can be expected to return in several weeks with the advent of cooler and wetter conditions. You could lightly prune off the recent lush leader growth, thereby delaying the start of new seasonal development by a month or so. As a last resort chemical treatments with fungicides such as Mancozeb or Triforine can assist control. Relatively minor attacks may occur on a few of the pink-flowered species such as Verticordia piumosa or Verticordia monadelpha but these will grow out later as weather conditions become cooler and wetter.
In near-coastal, summer-wet areas, Verticordia mitchelliana almost invariably suffers debilitation with serious leaf-drop in late summer. In inland districts the problem is rarely evident. The leaf-drop noted is usually rapid and comes at the end of a strong summer growth period during which the leaves have appeared almost succulent. The problem follows a change in weather pattern to cooler, less humid conditions and appears to start with fungal attack at leaf junctions with the stems The leaf drop is slightly less severe when the seasonal weather change is gradual rather than abrupt. Treatment with fungicides has failed.
All three species in Verticordia Section chrysorrhoe, viz. V. nitens, V. aurea and V. patens are particularly susceptible to foliar fungal attack. For V.nitens it can be extremely debilitating leading to rapid total collapse of the specimen, and such loses are not restricted to summer-wet areas. The natural growth habit is to produce very tall, fast-growing but soft-wooded flower stems. These stems are very susceptible to attack, the first indication of which is a purplish discoloration of the new growth a few inches below the growing tip. The only counter seems to be early pruning of rapidly growing soft-wooded stems to induce multiple branching. The plant form is then changed to that of a multi-branched lower shrub, which although still susceptible to fungal attack, is considerably more tenacious. In summer-wet areas the fungal attack is generally still severe enough to present a debilitated appearance. Verticordia aurea and V. patens, although not so badly affected as the above, are still difficult species to maintain, especially in summer-wet areas. The fungal attack occurs predominantly on the new leaves and stems causing severe debilitation with stem purpling.
Planting procedure
Potting mixes are very different in texture, nutrient value and moisture holding capacity from the garden soils that will receive the plant. Pre-planting preparation of Verticordia plants has been used to advantage for location compatibility and to provide greater resistance to root and collar rotting pathogens. Harden the plant in a pot of soil similar to that of its intended location. For advanced purchased plants, they are first bare-rooted, any root coiling removed and then lightly pruned. After repotting they should be staged in favourable conditions until new vigorous growth develops. Some additional measures have been used to advantage. The soil is not pre-cultivated but rather left in the compacted state. This is important for heavy garden conditions. Use a minimum sized hole, water the plant well. Autumn planting is preferred so that subsequent additional watering is not required. The plant is then given a hard prune. Follow-up treatment is restricted to removal of leggy growth. Verticordia monadelpha, V. attenuata and V. pholidophylla have responded well to this treatment in heavy base soils.
Cultivation of Verticordia
The information in the following table is based on experience in the eastern Australian, summer-wet conditions.
Key to soil type: Note: Where more than one soil type is shown, they are listed in order of preference, with a suffix ‘b’ for the most preferred soil.
- H: Heavy clay-loam.
- M: Medium finely-textured loam.
- L: Deep light sand.
- G: Predominantly gravel, deep with loamy-sand matrix.
- S: Sandstone parentage with high percentage of sandstone rubble.
- HL: Heavy-loam base with sand mixed into top 18 cm (medium depth root development).
- LM: Deep sand with medium loam mixed into top 18 cm (for deep root development).
Key to susceptibility to root rot, collar rot and to mould, etc.
- nil: No problem recorded.
- PI: Light attacks noted.
- Pm: Moderate attacks.
- Ps: Severe attacks noted.
Key to root stocks for grafted plants:
- D: Darwinia citriodora
- C: Chamelaucium uncinatum
Verticordia Cultivation Data
|
Species
|
Best Soil Type
|
Root Rot
|
Collar Rot
|
Mould
|
Grafted On To
|
|---|---|---|---|---|---|
|
V. acerosa var. acerosa
|
Gb, L, HL
|
nil
|
nil
|
PI
|
D, C
|
|
V. acerosa var. preissii
|
Mb, HL
|
nil
|
nil
|
nil
|
|
|
V. albida
|
Lb
|
Pl
|
Pm
|
nil
|
C
|
|
V. aereiflora
|
|||||
|
V. amphigia
|
|||||
|
V. apecta
|
|||||
|
V. argentea
|
|||||
|
V. attenuata
|
Gb, HLb, L, S
|
nil
|
nil
|
nil
|
C
|
|
V. aurea
|
HLb
|
nil
|
Pm
|
Ps
|
|
|
V. auriculata
|
Lb, HL
|
Pm
|
Pm
|
nil
|
|
|
V. bifimbriata
|
Lb
|
nil
|
nil
|
nil
|
|
|
V. blepharophylla
|
Gb
|
nil
|
nil
|
nil
|
D,C
|
|
V. brachypoda
|
Mb, HLb, H
|
PI
|
PI
|
nil
|
|
|
V. brevifolia ssp. brevifolia
|
Lb
|
nil
|
Pm
|
nil
|
|
|
V. brownii
|
HLb, L
|
Pm
|
PI
|
PI
|
D
|
|
V. capillaris
|
|||||
|
V. carinata
|
|||||
|
V. centipeda
|
Lb, HL
|
nil
|
Pm
|
nil
|
|
|
V. chrysantha
|
HLb, L
|
nil
|
PI
|
PI
|
|
|
V. chrysanthella
|
Hg, Lg, HLg, M
|
nil
|
nil
|
Pm
|
D
|
|
V. chrysostachys var. chrysostachys
|
HLb
|
PI
|
Pm
|
nil
|
C
|
|
V. chrysostachys var. pallida
|
Mb, LM
|
Ps
|
Pm
|
nil
|
C
|
|
V. citrella
|
Mb
|
nil
|
Pm
|
nil
|
|
|
V. comosa
|
|||||
|
V. cooloomia
|
HLb, H
|
PI
|
nil
|
PI
|
C, D
|
|
V. coronata
|
|||||
|
V. crebra
|
HLb, L
|
nil
|
nil
|
nil
|
|
|
V. cunninghamii
|
|||||
|
V. dasystylis ssp. oestopoia
|
HLb
|
nil
|
nil
|
nil
|
|
|
V. decussata
|
|||||
|
V. densiflora var. densiflora
|
LBb, Mb, HLb, H
|
nil
|
nil
|
nil
|
D
|
|
V. densiflora var. cespitosa
|
HLb, H
|
nil
|
PI
|
nil
|
|
|
V. dichroma var. dichroma
|
Lb
|
PI
|
Pm
|
nil
|
C
|
|
V. dichroma var. syntoma
|
Lb
|
nil
|
Pm
|
nil
|
C
|
|
V. drummondii
|
HLb ,L
|
nil
|
nil
|
nil
|
D, C
|
|
V. endlicheriana var. angustifolia
|
HLb
|
nil
|
nil
|
nil
|
|
|
V. endlicheriana var. compacta
|
Lb, G
|
nil
|
PI
|
nil
|
|
|
V. eriocephala
|
Lb
|
nil
|
nil
|
nil
|
|
|
V. etheliana var. etheliana
|
HLb, L
|
nil
|
nil
|
nil
|
C
|
|
V. euradyensis
|
|||||
|
V. fastigiata
|
Hb, HL
|
nil
|
nil
|
nil
|
D
|
|
V. fimbrilepis ssp. fimbrilepis
|
Gb
|
nil
|
PI
|
nil
|
|
|
V. forrestii
|
|||||
|
V. fragrans
|
Mb, G, HL
|
nil
|
nil
|
nil
|
D, C
|
|
V. galeata
|
D
|
||||
|
V. gracilis
|
|||||
|
V. grandiflora
|
Lb
|
nil
|
Pm
|
nil
|
D, C
|
|
V. grandis
|
Gb, HLb
|
nil
|
nil
|
nil
|
C
|
|
V. habiantha
|
Gb, HL
|
nil
|
PI
|
nil
|
|
|
V. halophila
|
LHb, M
|
nil
|
nil
|
nil
|
|
|
V. harveyi
|
L
|
nil
|
Ps
|
nil
|
|
|
V. helichrysantha
|
HLb
|
PI
|
nil
|
Ps
|
D, C
|
|
V. helmsii
|
|||||
|
V. huegelii var. huegelii
|
Hb. HL
|
nil
|
nil
|
nil
|
|
|
V. huegelii var. decumbens
|
Hb, HIb
|
nil
|
nil
|
nil
|
|
|
V. hughanii
|
Mb, HL, L, LM
|
Pm
|
PI
|
nil
|
|
|
V. humilis
|
HLb
|
nil
|
nil
|
nil
|
|
|
V. inclusa
|
|||||
|
V. insignis ssp. insignis
|
Gb, HL
|
PI
|
PI
|
PI
|
C
|
|
V. insignis ssp. compta
|
HLb
|
nil
|
PI
|
nil
|
|
|
V. integra
|
|||||
|
V. interioris
|
Lb
|
nil
|
Pm
|
nil
|
|
|
V. jamiesonii
|
|||||
|
V. laciniata
|
|||||
|
V. lehmannii
|
HLb, L
|
nil
|
Pm
|
Ps
|
D
|
|
V. lepidophylla var. lepidophylla
|
HLb, G, L
|
nil
|
nil
|
nil
|
|
|
V. lindleyi ssp. lindleyi
|
HLb, L
|
nil
|
nil
|
nil
|
D, C
|
|
V. lindleyi ssp. purpurea
|
Gb, L
|
PI
|
nil
|
nil
|
|
|
V. longistylis
|
Hb, HLb, Mb
|
nil
|
nil
|
nil
|
D
|
|
V. luteola
|
Gb, L
|
nil
|
nil
|
nil
|
|
|
V. minutiflora
|
Hb, HL
|
nil
|
nil
|
nil
|
|
|
V. mitchelliana
|
Sb, G, HL
|
Pm
|
nil
|
Ps
|
D
|
|
V. mitodes
|
|||||
|
V. monadelpha var. monadelpha
|
Gb, HL, S, LH
|
nil
|
nil
|
PI
|
D
|
|
V. monadelpha var. callitricha
|
Lb
|
nil
|
nil
|
nil
|
D
|
|
V. muelleriana ssp. muelleriana
|
Mb, L
|
nil
|
nil
|
nil
|
C
|
|
V. multiflora ssp. multiflora
|
HLb, L, S
|
PI
|
PI
|
nil
|
D
|
|
V. nitens
|
HLb
|
nil
|
nil
|
Ps
|
D
|
|
V. nobilis
|
HLb, G
|
nil
|
PI
|
nil
|
|
|
V. oculata
|
Lb
|
nil
|
PI
|
nil
|
D, C
|
|
V. ovalifolia
|
HLb, L
|
nil
|
Pm
|
nil
|
D, C
|
|
V. oxylepis
|
HLb, L
|
PI
|
nil
|
nil
|
D
|
|
V. paludosa
|
|||||
|
V. patens
|
Lb
|
nil
|
PI
|
Ps
|
|
|
V. penicillaris
|
Sb, L
|
nil
|
nil
|
PI
|
D
|
|
V. pennigera
|
Gb, HL, L
|
PI
|
nil
|
nil
|
C
|
|
V. pholidophylla
|
Hb, L
|
nil
|
nil
|
nil
|
C
|
|
V. picta
|
Lb, Gb
|
PI
|
nil
|
nil
|
C
|
|
V. pityrhops
|
|||||
|
V. plumosa var. plumosa
|
Lb, HLb, H
|
nil
|
nil
|
nil
|
D, C
|
|
V. plumosa var. ananeotes
|
HLb
|
nil
|
nil
|
nil
|
|
|
V. plumosa var. grandiflora
|
LMb, G
|
nil
|
nil
|
nil
|
|
|
V. plumosa var. pleiobotrya
|
Gb, HL
|
nil
|
nil
|
nil
|
|
|
V. plumosa var. vassensis
|
Mb, L, G
|
Pm
|
Ps
|
nil
|
|
|
V. polytricha
|
HLb
|
nil
|
nil
|
nil
|
|
|
V. pritzelii
|
HLb
|
nil
|
PI
|
nil
|
D
|
|
V. pulchella
|
Sb, G, L
|
nil
|
nil
|
nil
|
D
|
|
V. rennieana
|
Lb
|
PI
|
nil
|
nil
|
D, C
|
|
V. roei ssp. roei
|
Gb, LMb
|
nil
|
Pm
|
PI
|
|
|
V. roei ssp. meiogona
|
Lb
|
nil
|
Pm
|
PI
|
|
|
V. rutilastra
|
|||||
|
V. serotina
|
|||||
|
V. serrata var. linearis
|
HLb
|
nil
|
Pm
|
nil
|
D
|
|
V. serrata var. ciliata
|
HLb
|
PI
|
Pm
|
nil
|
|
|
V.sieberi var.sieberi
|
HLb, G
|
PI
|
Pm
|
nil
|
|
|
V. spicata
|
D, C
|
||||
|
V. staminosa ssp. staminosa
|
Lb, G
|
PI
|
Pm
|
nil
|
D
|
|
V. staminosa ssp. cylindracea
|
Gb, HL, L, M, H
|
PI
|
PI
|
nil
|
|
|
V. stenopetala
|
|||||
|
V. subulata
|
|||||
|
V. tumida ssp. tumida
|
Gb, HL
|
PI
|
PI
|
nil
|
|
|
V. venusta
|
Lb
|
PI
|
Pm
|
nil
|
C
|
|
V. verticordina
|
|||||
|
V. verticillata
|
|||||
|
V. vicinella
|
Lb
|
nil
|
nil
|
nil
|
|
|
V. wonganensis
|
Calytrix and Verticordia Study Groups
The Calytrix Study Group was formed with the aim of studying the cultivation and propagation of the genus Calytrix, a member of the large myrtle family of plants (the Myrtaceae), so that more species could be seen in gardens. Although the Group is now closed, a number of its newsletters are available for download.
The Verticordia Study Group was formed with similar aims. Although it too is now closed, many of the Group’s regular informative newsletters are available for download.
Further information about these Study Groups, their activities and access to the newsletter archives can be found at the links below.
Chamelaucium Alliance – Further Information
Most books dealing with Australian native plants will contain useful information on the botany and horticulture of Chamelaucium and its relatives. Some of the most detailed references are listed below.
Books:
- Blake, T (1981), “A Guide to Darwinia and Homoranthus”, Society for Growing Australian Plants, Maroondah Group, Ringwood.
- Elliot, R and Jones D (1982 to current), “The Encyclopaedia of Australian Plants”, All volumes, Lothian Publishing Company Pty Ltd, Melbourne.
- George, E (2002), “Verticordia: Turner of Hearts”, University of Western Australia Press, Perth.
Journals:
- Bean AR (1995); “Reinstatement and revision of the genus Triplarina Raf. (Myrtaceae)”. Austrobaileya 4, 353-367.
- Bean AR (1997a); “Reinstatement of the genus Babingtonia Lindl. (Myrtaceae, Leptospermoideae)”. Austrobaileya 4, 627-645.
- Bean AR (1997b); “A revision of Baeckea (Myrtaceae) in eastern Australia, Malesia and south-east Asia”. Telopea 7, 245-268.
- Bean AR (1999); “A revision of the Babingtonia virgata (J.R.Forst. & G.Forst.) F.Muell. complex (Myrtaceae) in Australia”. Austrobaileya 5, 157-171.
- Wilson P.G., O’Brien M, Gadek P.A. and Quinn C.J (2001); “Myrtaceae revisited: a reassessment of infrafamilial groups”, American Journal of Botany, 88:2013-2025.
- Wilson P.G., Heslewood, M.M. and Quinn C.J (2007); “Re-evaluation of the genus Babingtonia (Myrtaceae) in eastern Australia and New Caledonia”, Australian Systematic Botany 20, 302-318,.
Several issues of the Society’s journal “Australian Plants” are particularly useful for those interested in Chamelaucium and related plants.
- Vol 1, No.3 June 1960; “Some Observations on the Genus Verticordia”.
- Vol 1, No.5 December 1960; “Try Verticordia from Seed”.
- Vol 1, No.6 March 1961; “Verticordia for the Garden”.
- Vol 3, No.23 June 1965; “Pileanthus; The ‘Copper Cups’ Genus”.
- Vol 4, No.30 March 1967; “Heath Myrtles; Thryptomene, Micromyrtus and Baeckea”.
- Vol 4, No.30 March 1967; “Baeckea”.
- Vol 4, No.30 March 1967; “Heath Myrtles; A Few Comparable Genera in the Family Myrtaceae”.
- Vol 4, No.31 June 1967; “Hypocalymma”.
- Vol 4, No.32 September 1967; “Waxflowers of W. A.”.
- Vol 4, No.32 September 1967; “Chamelauciums”.
- Vol 4, No.32 September 1967; “The Geraldton Wax”.
- Vol 9, Nos.69 and 70 December 1976 and March 1977; A two-part, detailed description of the myrtle family including a botanical outline of the features of the Myrtaceae.
- Vol 10, No.80 September 1979; “Verticordia – As I See Them”.
- Vol 12, No.95 June 1983; “Actinodium”.
- Vol 12, No.99 March 1984; “Verticordia – Studies in Propagation”
- Vol 14, No.110 March 1987; “Darwinia”
- Vol 14, No.111 June 1987; “Darwinia – Some Western Species”
- Vol 14, No.112 September 1987; Myrtles, including Malleostemon.
- Vol 14, No.113 December 1987; “Rinzia”
- Vol 15, No.123 June 1990; Most of this issue is dedicated to Calytrix
- Vol 17, No.134 March 1993; “Grafting the Grampians Thryptomene”
- Vol 18, No.145 December 1995; Most of this issue is dedicated to Verticordia
- Vol 18, No.146 March 1996; “Growing Darwinia – Grafted for Reliability”
- Vol 18, No.146 March 1996; “Darwinia”
- Vol 21, No.168 September 2001; “Darwinia”
- Vol 22, No.175 June 2003; “Domestication of ‘Golden Cascade’ (Corynanthera flava)”
- Vol 22, No.177 December 2003; “Coppercups; The Genus Pileanthus”
- Vol 22, No.179 June 2004; “Balaustion; Native Pomegranates”
Internet:
- Bringing Verticordia out of the Too Hard Basket
- Evolution of the Myrtle Family in Australia
- Growing Darwinia….Grafted for Reliability
- Growing Native Plants – a series of plant profiles by the Australian National Botanic Gardens; includes a number of species in the Chamelaucium alliance.
- Shrubby Myrtles
- Smoke Stimulates the Germination of Many Western Australian Plants, by K.Dixon and S.Roche – contains useful information on research into many plant genera including some of the Chamelaucium alliance.
- The Genus Hypocalymma
- Those Other Myrtaceae
- When is a Baeckea not a Baeckea?
 Australian Native Plants Society (Australia)
Australian Native Plants Society (Australia)
