Australian Daisies – Background
Daisies are members of the Asteraceae, a diverse family of plants comprising about 20,000 species worldwide. In Australia there are almost 1,000 indigenous species comprising shrubs, sub-shrubs, perennial herbs, annuals and a few biennials. There are too many Australian daisies to generalize about the growing conditions they enjoy. Some prefer full sun, others like shade, some like open conditions, others prefer overhead protection. They all appreciate group planting whereby they benefit from the increased possibilities for cross-pollination.
There are daisies for all soils from clay to sand, wet to dry, but usually improved growth results if the soil is well drained, enriched with fertilizers and slightly acid.
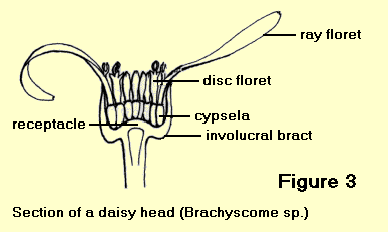
The apparent ‘flower’ of a daisy is a condensed inflorescence composed of a large number of small stalkless flowers arranged on an expanded stem called a ‘receptacle’. The inflorescence is known as a ‘capitulum’ or ‘head’, and each individual flower is called a ‘floret’.
Florets are of two main types:
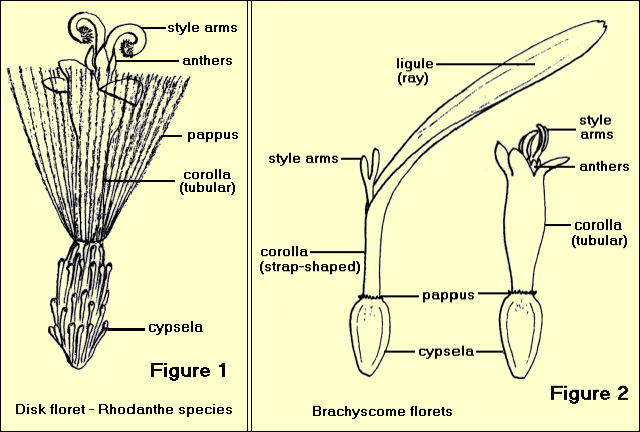
1. Tubular (or disc) florets have a regular corolla of 5 (sometimes 4) united petals with 5 (or 4) lobes. The calyx (if present) consists of a ring of hairs or scales known as the ‘pappus’. There are 5 stamens united by their anthers to form a tube around the style. The style is divided at the top into two ‘arms’. The ovary is ‘inferior’, (ie it occurs below the calyx) – Figure 1
2. Ligulate (or ray) florets have an irregular corolla of 5 (or 3) petals which are united at the base and then extend into a flat strap-shaped structure called a ‘ligule’ or ray which has 5 (or 3) small lobes. A pappus of bristles, scales or awns may be present. The stamens are united by their anthers to form a tube, the style is divided, and the ovary is inferior – Figure 2
The ligules are the apparent ‘petals’ in the typical daisy flowers such as Brachyscome, Olearia and Celmesia.
The structure of a daisy head depends on the arrangement of the two main types of florets.
- In ligulate heads all the florets are ligulate and 5-lobed and usually they are all bisexual. An example is the genus Microseris or ‘Yam Daisies’.
- Discoid heads have all tubular florets which may be all bisexual, or there may be female or sterile florets around the periphery of the head. An example is the genera Rhodanthe.
- Heads with both ligulate and tubular florets are called radiate heads. The tubular florets are usually bisexual and are found in the centre of the receptacle with the ligulate florets arranged around the outside in one or two rows. The ligulate florets are usually either female or sterile. An example is the genus Brachyscome – Figure 3.
Each head is generally surrounded by an ‘involucre’ of bracts which occur in several rows, and may be soft and herbaceous as in Brachyscome or stiff and papery as in Rhodanthe.
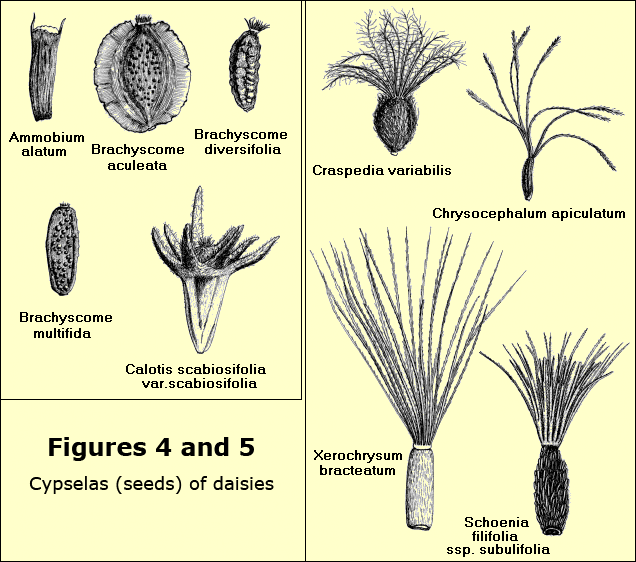
The fruit is dry, one-seeded and indehiscent (that is, it does not open to disperse its contents) and is known as a cypsela.
The criteria for identification are many but one of the most important is the appearance of the mature cypsela. Under a microscope the presence or absence of a pappus, whether it is feathery or barbed, the size, shape and colour of the body of the cypsela, whether it is smooth, hairy, warted or winged, all help with identification. Other criteria include the appearance of the receptacle, the bracts, anthers and style-arms as well as the characteristics of habit, leaf, stem and arrangement of heads – Figures 4 and 5.
Australian Daisies – Propagation
Introduction
Daisies may be propagated successfully from seed or vegetatively from cuttings, suckers or divisions from established plants. Vegetative methods should be used to preserve desirable characteristics of a particular plant or cultivar.
Seed
Daisy seed is easy to collect when the pappus is conspicuous, such as in Craspedia Chrysocephalum, and Rhodanthe species, but in genera such as Brachyscome and Ammobium where the pappus is inconspicuous, the dried ray florets must first fall off to reveal the mature seed on the receptacle. Collect seed from healthy vigorous plants when the weather is dry.
The majority of annuals are grown from seed. This may be sown directly in the soil or in seed trays. The medium should be well drained, capable of retaining air and moisture, and of a pH suitable for root initiation (4.5 to 5.5). A thin layer of sand, gravel or blue-metal chips over the seed prevents heavy rain from dislodging it. Sowing thinly allows more air movement around seedlings and discourages fungal attack such as ‘damping-off’.
Many daisies, particularly those from arid areas, have a mechanism known as ‘dormancy’ to prevent germination in unfavourable conditions. Many factors may be responsible for this dormancy, such as the immaturity of the embryo, or the presence of chemical inhibitors to germination. Dormancy in some species can be overcome by storing for a period at certain temperatures, a procedure known as ‘after-ripening’. Germination may also be improved by treatment with ‘smoke-water’ or gibberellin preparations. (For a summary of some of the research being undertaken into dormancy see information under the tab titled “Seed Germination Requirements”).
Cuttings, Suckers and Divisions
Stem cuttings of most perennials usually strike quickly and easily even with the simplest of equipment, provided vigorous healthy growth is selected. The medium should be an open free-draining mix which retains some moisture, such as 3 parts washed course sand to one part peat moss. Root stimulating hormones may be used to advantage.
Many of the perennial daisies produce suckers which may be simply cut off the parent plant and established in other parts of the garden or in pots. Examples are Brachyscome multifida, B.angustifolia, B.segmentosa. Others, such as Brachyscome stolonifera and Senecio pectinatus are stoloniferous, and these also are easy to separate and remove for planting elsewhere. Rosetted perennial daisies such as Brachyscome diversifolia, B.scapigera and B.nivalis may be propagated by simple division of clumps in late winter or early spring.
General Propagation
Further details on general plant propagation can be found at the Society’s Plant Propagation Pages.
Australian Daisies – Germination Requirements
David Murray
This article has been reproduced from the April 1995 issue of Native Plants for New South Wales, newsletter of the Australian Plants Society (NSW).

Photo: Australian Daisy Study Group
Australian daisies are potentially very popular as ornamental pot plants. To help realise their full potential, a systematic evaluation of seed germination requirements was needed, as well as other information on vegetative propagation and flowering requirements.
In 1992, the Australian Flora Foundation provided a grant to help Dr K.V.Sharman and Dr R.M.Dowling at the Queensland Department of Primary Industries, Redlands Research Station, study the germination requirements of more than 20 species. They later secured further funding from HRDC (Horticultural Research and Development Corporation) to study flowering requirements.
Selection of Species
Because of the drought, a planned field trip to western Queensland to collect seed of species of interest was not undertaken. Instead, seed was obtained from commercial suppliers and volunteer donors from the Australian Daisy Study Group.
Germination Tests
The seed to be tested was first stored at room temperature for a minimum of 6 months, to eliminate any possible dormancy due to a requirement for a period of ‘after-ripening’. Between three and six species were included in each germination trial, there being five trials altogether. Tests were carried out using 9 cm Petri dishes lined with filter papers. For each species evaluated, the treatments were:
- intact seed incubated with water,
- scarified seed (pierced with dissecting needles) incubated with water, and
- intact seed incubated with gibberellic acid (GA3) at 500 mg per litre.
The fungicide Thiram (0.2 %) was included in both the water and the GA3 solution, dispensed at 5 ml per dish. For each treatment, there were five replicate dishes, each containing 15 seeds. The dishes were placed under ambient conditions with either a light/dark cycle, or continuous darkness (achieved by wrapping in alfoil).
The seeds of one species, Chrysocephalum apiculatum, were too small to scarify without damaging the embryo, hence this treatment was omitted. As the seeds were not dormant (see below), the absence of this treatment did not matter.
Emergence of the radicle was taken to indicate germination. Seeds that had germinated were counted every 3 days for 30 days, or at day 15 only for the dark treatments. At the end of the assessment period, ungerminated seeds were dissected, and those that contained partially formed or no embryos were scored as non-viable. Germination was then expressed on a viable seed basis.
 |
 |
|
| Left: Chrysocephalum apiculatum Right: Schoenia filifolia subsp. subulifolia Photos: Australian Daisy Study Group |
||
Non-dormant species
Five species showed no dormancy and were not stimulated by light: Brachyscome latisquaemea, Podolepis gracilis, Rhodanthe chlorocephala subsp. rosea (syn. Helipterum roseum), Rhodanthe humboldtiana (syn. Helipterum humboldtianum), and Schoenia filifolia subsp. subulifolia (syn. Helichrysum subulifolium).
Four more showed no dormancy and were stimulated by light, although light was not an absolute requirement for germination: Brachyscome iberidifolia, Chrysocephalum apiculatum, Hyalosperma glutinosum subsp. venustum (syn. Helipterum venustum), and Watsia acuminata.
Dormant species

Photo: Australian Daisy Study Group
One species, Lawrencella rosea (syn. Helichrysum lindleyii) showed complete dormancy, overcome by scarification. Another two species showed complete dormancy, overcome by GA3: Chrysocephalum podolepidium and Leucochrysum molle (syn. Helipterum molle).
Altogether, nine other species showed partial dormancy. For two of these, Rhodanthe chlorocephala subsp. chlorocephala (syn. Helipterum chlorocephalum) and Rhodanthe manglesii (syn. Helipterum manglesii), scarification alone was sufficient to promote germination; and for one, Leucochrysum stipitatum (syn. Helipterum stipitatum) either scarification or GA3was effective.

Photo: Australian Daisy Study Group
The remaining six species all responded to GA3: Leucochrysum fitzgibbonii (syn. Helipterum fitzgibbonii), Myriocephalus stuartii, Podolepis jaceoides, Rhodanthe moschata (syn. Helipterum moschata), Rhodanthe polygalifolia (syn. Helipterum polygalifolium), and Rhodanthe stricta (syn. Helipterum stricta).
Failure to germinate
Seeds failed to germinate for one species under all circumstances: Erymophyllum ramosum subsp. involucratum (syn. Helipterum involucratum) .
Conclusion Regarding Depth of Planting
As a general rule those species with seeds responding to a light stimulus should be sown on the soil surface, with all others sown beneath a shallow cover.
Future Dormancy Assessment
Dr Sharman and Dr Dowling concluded that it would be worthwhile to test responses to a higher temperature during seed storage. Of interest also are observations from the Kirstenbosch Botanic Garden in South Africa showing that the compounds extracted from bushfire smoke have the ability to overcome dormancy in a variety of species, including the former genus Helichrysum (as reported in The Australian Garden Journal, 1995).
Horticultural Potential
A panel of seventeen retailers and wholesalers have judged the horticultural potential of more than 40 species and varieties cultivated at Redlands Research Station. Species were preferred on the basis that they had compact upright growth (less than 35 cm tall), and produced numerous small flowers, or several distinctive showy blooms.
The top nine, in rank order, were:
- Rhodanthe manglesii
- Rhodanthe floribunda
- Schoenia filifolia subsp. subulifolia
- Brachyscome halophila
- Lawrencella davenportii
- Schoenia cassiniana
- Hyalosperma cotula
- Lawrencella rosea
- Hyalosperma glutinosum subsp. venustum
It is gratifying that four of the top nine species, including those ranked first and third, had been included in this germination study.
Australian Daisies – Cultivation
The diversity of daisies, the extent of their flowering periods, the delicacy and charm of their flowers and their generally small dimensions all combine to ensure that they find a place in every well-designed garden.
They can be used in many ways in gardens: massed as bedding plants, in hanging baskets, as ground covers, as edging plants, in rockeries, lining pools, as bog plants, for grouping or for splashes of colour in pockets among existing shrubs. Larger species are useful as specimen or background shrubs or for foliage contrast.
Annuals (or fast growing perennials) can be used to fill the empty spaces between permanent plantings in new gardens. There are daisies for coastal, alkaline, alpine, or arid situations. However, in tropical and subtropical gardens high humidity, the force of tropical rain and root rot fungi in the soil can combine to cause the death of daisies.
Cultivation in containers of any kind has many benefits; the right soil type can be provided, the containers can be moved to an advantageous position. A suitable soil mix is 3 parts friable soil, 4 parts organic material (peat moss, leaf mould or compost) and 5 parts course washed sand. The pH should be adjusted to 6 and trace elements and fertilizers added. This basic mix can be varied to cater for the requirements of a particular species. For example alpine species such as Celmisia asteliifolia benefit from the addition of extra peat moss.
Small amounts of slow release fertilizer should be used in spring and autumn to maintain healthy growth.
 |
 |
|
| Left: Xerochrysum bracteatum; Mixed colours Right: Olearia phlogopappa – white form Photos: Brian Walters |
||
Daisies are very useful for floral art and in this context fall into two categories. The first, those capable of being dried, includes the ‘everlastings’ or ‘paper daisies’. They have stiff, papery, colourful bracts surrounding the flower heads. These bracts retain their shape and colour when dried. Examples are Ammobium, Cephalipterum, Ixodia, Rhodanthe and Waitzia species. Others, which dry well but have inconspicuous bracts, include Calocephalus and some Craspedia species. Drying may be achieved by simply hanging loose bunches upside-down in an airy place out of direct sunlight. The heads of many everlastings may be wired if the stem is not too narrow.
The second group, have typical ‘daisy flowers’ with ligulate florets (see the tab titled “Background”) and shorter soft green bracts (resembling the calyx of a single flower). This group cannot be dried, but usually have a long vase life as fresh cut flowers. Examples are Brachyscome, Olearia and Senecio species. The usual methods of increasing the lasting qualities of cut flowers apply to daisies.
Australian Daisy Study Group
This Study Group was formed in 1981 with the aim of studying the cultivation and propagation of Australia’s native daisies – the family Asteraceae. Although this Study Group is now closed, during its period of operation the Group produced a series of highly detailed newsletters documenting reports from members into cultivation issues, propagation methods, natural occurrences of different species and taxonomy.
Access to the group’s newsletters can be found at the following link. Also available are downloadable versions of the three books produced by the group.
Australian Daisies – Further Information
Three books have been published by the Australian Daisy Study Group specifically on Australian daisies. In addition, most books dealing with Australian native plants will contain useful information on the botany and horticulture of at least some species of Australian native daisies. There are also a number of resources to Australian daisies on the internet. Some of the most detailed references are listed below.
Books:
- Australian Daisy Study Group (1995), Australian Brachyscomes, Australian Daisy Study Group.*
- Insert to ‘Australian Brachyscomes’: Chart of Brachyscome Fruits
- Australian Daisy Study Group (1987), Australian Daisies for Gardens and Floral Art, Lothian.*
- Australian Daisy Study Group (2002), Everlasting Daisies of Australia, C.H. Jerram & Associates.*
- Elliot, R and Jones D (1980-1997), The Encyclopaedia of Australian Plants, all volumes, Lothian Publishing Company Pty Ltd, Melbourne.
- Wrigley, J and Fagg, M (1996 – 4th ed), Australian Native Plants, Collins Publishers Australia.
* Electronic version included with the permission of the authors
Journals:
Several issues of the Society’s journal “Australian Plants” are particularly useful for those interested in Australian daisies, in particular:
- Vol 2, No.19 June 1964; The Compositae or Daisy Family – although now outdated, very useful botanical details of the family.
- Vol 7, No.60, September 1974; Native daisies of alpine areas.
- Vol 15, No.124, September 1990; The Golden everlasting.
- Vol 17, No.140, September 1994; Australian daisies that have proven reliable in cultivation; Brachyscome and Rhodanthe cultivars.
- Vol 19, No.155, June 1998; The daisy family; Ozothamnus diosmifolius as a commercial cut flower; Daisies as potted plants; Ixodia; Acomis, a tropical daisy.
- Vol.21 No.167 June 2001; Pink everlasting daisies – a breeder’s perspective.
- Vol.21 No.168 September 2001; Daisies and a wedding.
- Vol.21 No.171 June 2002; Bracteantha micropropagation.
- Vol.21 No.173 December 2002; Xerochrysum – the correct name for the genus Bracteantha; Olearia viscosa.
- Vol 22, No.178 March 2004; “Pink Everlasting Daisy; Is pollination wind-assisted?”
Internet:
- Australian Daisies.
- The Genus Olearia.
- The Golden Everlasting.
- Growing Native Plants – a series of plant profiles by the Australian National Botanic Gardens; includes a number of species in the Asteraceae.
- Olearia – The Daisy Bush – a short item on growing and propagating Olearia.
 Australian Native Plants Society (Australia)
Australian Native Plants Society (Australia)
